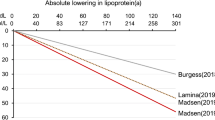
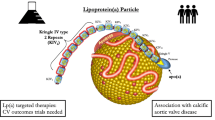
Abstract
Purpose of Review
Lipoprotein(a) is an important causal risk factor for cardiovascular disease but currently no available medication effectively reduces lipoprotein(a). This review discusses recent findings regarding lipoprotein(a) as a causal risk factor and therapeutic target in cardiovascular disease, it reviews current clinical recommendations, and summarizes new lipoprotein(a) lowering drugs.
Recent Findings
Epidemiological and genetic studies have established lipoprotein(a) as a causal risk factor for cardiovascular disease and mortality. Guidelines worldwide now recommend lipoprotein(a) to be measured once in a lifetime, to offer patients with high lipoprotein(a) lifestyle advise and initiate other cardiovascular medications. Clinical trials including antisense oligonucleotides, small interfering RNAs, and an oral lipoprotein(a) inhibitor have shown great effect on lowering lipoprotein(a) with reductions up to 106%, without any major adverse effects.
Summary
Recent clinical phase 1 and 2 trials show encouraging results and ongoing phase 3 trials will hopefully result in the introduction of specific lipoprotein(a) lowering drugs to lower the risk of cardiovascular disease.

Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Utermann G. The mysteries of lipoprotein(a). Science. 1989;246:904–10. https://doi.org/10.1126/science.2530631.
• Nissen SE, Wolski K, Cho L, et al. Lipoprotein(a) levels in a global population with established atherosclerotic cardiovascular disease. Open Heart. 2022;9:e002060. https://doi.org/10.1136/openhrt-2022-002060. Large multicentre study describing differences in Lp(a) levels across different populations with atherosclerotic cardiovascular disease.
Simony SB, Mortensen MB, Langsted A, Afzal S, Kamstrup PR, Nordestgaard BG. Sex differences of lipoprotein(a) levels and associated risk of morbidity and mortality by age: The Copenhagen General Population Study. Atherosclerosis. 2022;355:76–82. https://doi.org/10.1016/j.atherosclerosis.2022.06.1023.
Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28. https://doi.org/10.1056/NEJMoa0902604.
Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–9. https://doi.org/10.1001/jama.2009.801.
Collaboration ERF. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. https://doi.org/10.1001/jama.2009.1063.
Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12. https://doi.org/10.1056/NEJMoa1109034.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–7. https://doi.org/10.1016/j.jacc.2013.09.038.
Kamstrup PR, Nordestgaard BG. Elevated Lipoprotein(a) Levels, LPA Risk Genotypes, and Increased Risk of Heart Failure in the General Population. JACC Heart Fail. 2016;4:78–87. https://doi.org/10.1016/j.jchf.2015.08.006.
Langsted A, Kamstrup PR, Nordestgaard BG. High Lipoprotein(a) and low risk of major bleeding in brain and airways in the general population: A Mendelian randomization study. Clin Chem. 2017;63:1714–23. https://doi.org/10.1373/clinchem.2017.276931.
Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40:2760–70. https://doi.org/10.1093/eurheartj/ehy902.
Wang ZW, Li M, Li JJ, Liu NF. Association of lipoprotein(a) with all-cause and cause-specific mortality: A prospective cohort study. Eur J Intern Med. 2022;106:63–70. https://doi.org/10.1016/j.ejim.2022.09.010.
Levin MG, Zuber V, Walker VM, et al. Prioritizing the role of major lipoproteins and subfractions as risk factors for peripheral artery disease. Circulation. 2021;144:353–64. https://doi.org/10.1161/CIRCULATIONAHA.121.053797.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1732–41. https://doi.org/10.1161/ATVBAHA.112.248765.
Di Maio S, Lamina C, Coassin S, et al. Lipoprotein(a) and SARS-CoV-2 infections: Susceptibility to infections, ischemic heart disease and thromboembolic events. J Intern Med. 2022;291:101–7. https://doi.org/10.1111/joim.13338.
Jackson CL, Garg PK, Guan W, et al. Lipoprotein(a) and coronary artery calcium in comparison with other lipid biomarkers: the multi-ethnic study of atherosclerosis. J Clin Lipidol. 2023. https://doi.org/10.1016/j.jacl.2023.06.002.
Kaiser Y, Daghem M, Tzolos E, et al. Association of Lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol. 2022;79:223–33. https://doi.org/10.1016/j.jacc.2021.10.044.
Chandra S, Nagar S, Shukla A, et al. Correlation of lipoprotein (a) levels and plaque morphology in very young acute coronary syndrome patients using optical coherence tomography. Indian Heart J. 2022;74:357–62. https://doi.org/10.1016/j.ihj.2022.09.001.
van der Valk FM, Bekkering S, Kroon J, et al. Oxidized Phospholipids on Lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–24. https://doi.org/10.1161/CIRCULATIONAHA.116.020838.
Tsimikas S, Duff GW, Berger PB, et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J Am Coll Cardiol. 2014;63:1724–34. https://doi.org/10.1016/j.jacc.2013.12.030.
Naka KK, Bechlioullis A, Marini A, et al. Interleukin-1 genotypes modulate the long-term effect of lipoprotein(a) on cardiovascular events: the Ioannina Study. J Clin Lipidol. 2018;12:338–47. https://doi.org/10.1016/j.jacl.2017.12.004.
Puri R, Nissen SE, Arsenault BJ, et al. Effect of C-Reactive protein on Lipoprotein(a)-Associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE trial. JAMA Cardiol. 2020;5:1136–43. https://doi.org/10.1001/jamacardio.2020.2413.
Wang Y, Wang Z, Yang Q, et al. Autosomal recessive transmission of MYBPC3 mutation results in malignant phenotype of hypertrophic cardiomyopathy. PLoS ONE. 2013;8:e67087. https://doi.org/10.1371/journal.pone.0067087.
Thomas PE, Vedel-Krogh S, Kamstrup PR, Nordestgaard BG. Lipoprotein(a) is linked to atherothrombosis and aortic valve stenosis independent of C-reactive protein. Eur Heart J. 2023;44:1449–60. https://doi.org/10.1093/eurheartj/ehad055.
O’Donoghue ML, Morrow DA, Tsimikas S, et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J Am Coll Cardiol. 2014;63:520–7. https://doi.org/10.1016/j.jacc.2013.09.042.
Verbeek R, Hoogeveen RM, Langsted A, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J. 2018;39:2589–96. https://doi.org/10.1093/eurheartj/ehy334.
• O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–92. https://doi.org/10.1161/CIRCULATIONAHA.118.037184. Post-hoc analyses of the FOURIER trial showing that in secondary prevention Lp(a) associates with cardiovascular risk, and that degree of Lp(a) reduction when receiving evolocumab is associated with the degree of risk reduction.
• Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein(a)-Lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol. 2020;40:255–66. https://doi.org/10.1161/ATVBAHA.119.312951. Large cohort study estimating the needed Lp(a) lowering effect to achieve a 20% cardiovascular risk reduction in secondary prevention as well as showing that the relative cardiovascular risk associated of Lp(a) is similar for individuals with LDL cholesterol below 1.8 mmol/L and above 2.6 mmol/L.
• Langsted A, Nordestgaard BG, Kamstrup PR. Low lipoprotein(a) levels and risk of disease in a large, contemporary, general population study. Eur Heart J. 2021;42:1147–56. https://doi.org/10.1093/eurheartj/ehaa1085. Large cohort study finding no increased risk of cancer, infections, respiratory, or endocrine diseases associated with low Lp(a) compared to high Lp(a).
Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010;56:1252–60. https://doi.org/10.1373/clinchem.2010.146779.
Kamstrup PR, Nordestgaard BG. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2013;1:220–7. https://doi.org/10.1016/S2213-8587(13)70064-0.
Gudbjartsson DF, Thorgeirsson G, Sulem P, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74:2982–94. https://doi.org/10.1016/j.jacc.2019.10.019.
Tolbus A, Mortensen MB, Nielsen SF, Kamstrup PR, Bojesen SE, Nordestgaard BG. Kringle IV Type 2, not low Lipoprotein(a), as a cause of diabetes: a novel genetic approach using SNPs associated selectively with Lipoprotein(a) concentrations or with Kringle IV Type 2 repeats. Clin Chem. 2017;63:1866–76. https://doi.org/10.1373/clinchem.2017.277103.
Chasman DI, Shiffman D, Zee RY, et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–6. https://doi.org/10.1016/j.atherosclerosis.2008.07.019.
• Lacaze P, Bakshi A, Riaz M, et al. Aspirin for primary prevention of cardiovascular events in relation to Lipoprotein(a) genotypes. J Am Coll Cardiol. 2022;80:1287–98. https://doi.org/10.1016/j.jacc.2022.07.027. Post hoc study of the ASPREE randomized clinical trial of older individuals without cardiovascular disease. Showed that individuals with genetically elevated Lp(a) due to the rs3789220 variant receiving aspirin 100 mg daily did not have increased risk of major cardiovascular events compared with individuals without genetically elevated Lp(a), whereas those with genetically elevated Lp(a) not receiving aspirin did have increased risk.
McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–18. https://doi.org/10.1056/NEJMoa1805819.
•• Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455. The latest European guidelines includes recommendations to meassure Lp(a) once in every individuals life to asses cardiovascular risk.
Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–50. https://doi.org/10.1016/j.cjca.2021.03.016.
• Hedegaard BS, Bork CS, Kaltoft M, et al. Equivalent impact of Elevated Lipoprotein(a) and Familial Hypercholesterolemia in patients with atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2022;80:1998–2010. https://doi.org/10.1016/j.jacc.2022.09.021. Large cohort study estimating Lp(a) equivalents to familial hypercholesterolemia with regards to risk of myocardial infarction and atherosclerotic cardiovascular disease.
Bittner VA, Szarek M, Aylward PE, et al. Effect of Alirocumab on Lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–44. https://doi.org/10.1016/j.jacc.2019.10.057.
Szarek M, Bittner VA, Aylward P, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41:4245–55. https://doi.org/10.1093/eurheartj/ehaa649.
•• Kronenberg F, Mora S, Stroes ESG, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925–46. https://doi.org/10.1093/eurheartj/ehac361. Comprehensive consensus statement on Lp(a) with focus on atherosclerotic cardiovascular disease and aortic stenosis.
Safarova MS, Moriarty PM. Lipoprotein Apheresis: current recommendations for treating familial hypercholesterolemia and elevated Lipoprotein(a). Curr Atheroscler Rep. 2023;25:391–404. https://doi.org/10.1007/s11883-023-01113-2.
Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–53. https://doi.org/10.1016/S0140-6736(16)31009-1.
•• Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–55. https://doi.org/10.1056/NEJMoa1905239. Phase 2 results from randomized controlled trial of the antisense oligonucleotide APO(a)-LRx (now named pelacarsen) showing a Lp(a) reduction up to 80% from baseline with no adverse effects.
Karwatowska-Prokopczuk E, Lesogor A, Yan JH, et al. Efficacy and safety of pelacarsen in lowering Lp(a) in healthy Japanese subjects. J Clin Lipidol. 2023;17:181–8. https://doi.org/10.1016/j.jacl.2022.12.001.
•• O’Donoghue ML, Rosenson RS, Gencer B, et al. Small interfering RNA to reduce Lipoprotein(a) in cardiovascular disease. N Engl J Med. 2022;387:1855–64. https://doi.org/10.1056/NEJMoa2211023. Phase 2 results from a randomized controlled trial of the small interfering RNA olpasiran showing a Lp(a) reduction up to 98% from baseline with no adverse effects.
Rider DA, Eisermann M, Loffler K, et al. Pre-clinical assessment of SLN360, a novel siRNA targeting LPA, developed to address elevated lipoprotein (a) in cardiovascular disease. Atherosclerosis. 2022;349:240–7. https://doi.org/10.1016/j.atherosclerosis.2022.03.029.
Nissen SE, Wolski K, Balog C, et al. Single ascending dose study of a short interfering RNA targeting Lipoprotein(a) production in individuals with elevated plasma Lipoprotein(a) levels. JAMA. 2022;327:1679–87. https://doi.org/10.1001/jama.2022.5050.
•• Nicholls SJ, Nissen SE, Fleming C, et al. Muvalaplin, an oral small molecule inhibitor of Lipoprotein(a) formation: a randomized clinical trial. JAMA. 2023. https://doi.org/10.1001/jama.2023.16503. Phase 1 results from a randomized controlled trial of the once per day orally administered small molecule inhibitor of Lp(a) called muvalaplin. In individuals receiving muvalaplin Lp(a) were reduced up to 65% after 14 days of therapy.
Acknowledgements
We thank the staff and participants from the Copenhagen General Population Study for their valuable contributions.
Funding
Novo Nordisk Foundation (grant NNF21OC0071977) (ABW), Aase and Ejnar Danielsen Fund (BGN), Independent Research Fund Denmark (BGN). The funders had no role in the design of the study or in the collection, analysis, interpretation of data, writing the manuscript, or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
ABW performed the literature search and wrote the first draft of the manuscript. ABW, BGN, and AL revised the manuscript for critical scientific content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
BGN reports consultancies and talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Ionis, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, Abbott, Silence Therapeutics, Mankind, Ultragenyx, and Lilly. AL reports a talk sponsored by Amarin. ABW does not have any financial competing interests.
Human/Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Employment
Recent, present, and anticipated employment is The Capital Region (Danish: Region Hovedstaden) in Denmark for all authors, all paid through tax-generated money without any influence from private companies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wulff, A.B., Nordestgaard, B.G. & Langsted, A. Novel Therapies for Lipoprotein(a): Update in Cardiovascular Risk Estimation and Treatment. Curr Atheroscler Rep 26, 111–118 (2024). https://doi.org/10.1007/s11883-024-01192-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-024-01192-9