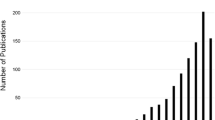
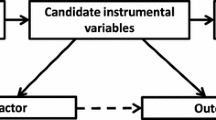
Abstract
Genetic factors identified from genome-wide association studies have been used to understand causative variants for complex diseases. Studies conducted on large populations of individuals from many geographical regions have provided insights into genetic pathways involved in the causal pathway for atherosclerotic cardiovascular disease. A single genetic trait may ineffectively evaluate the pathway of interest, and it may not account for other complementary genetic pathways that may be activated at various stages of the disease process or evidence-based therapies that alter the molecular and cellular milieu.
Similar content being viewed by others
References
DeLoukas P, Kanoni S, Willenborg C, for the CARDioGRAMplusD4D consortium, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33.
Roberts R. Genetics of coronary artery disease. Circ Res. 2014;114:1890–903.
Rosenson RS. Future role of selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther. 2009;23:93–101.
Schradt EE, Bjorkegren JL. NEW: network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
Bjorkegren JLM, Kovacic JC, Dudley JT, Schadt EE. Genome-wide significant loci: how important are they? J Am Coll Cardiol. 2015;65:830–45.
Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29:722–9.
Ridker PM, Paynter NP, Danik JS, Glynn RJ. Interpretation of Mendelian randomization studies and the search for causal pathways in atherothrombosis: the need for caution. Metab Syndr Relat Disord. 2010;8:465–9.
Sofat R, Hingorani AD, Smeeth L, et al. Separating the mechanism-based and off-target actions of cholesteryl ester transfer protein inhibitors with CETP gene polymorphisms. Circulation. 2010;121:52–62.
McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–91.
Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–53.
Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–3.
Kathiresan S, Voight BF, Purcell S, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41(3):334–41.
Do R, Stitziel NO, Won HH, Jørgensen AB, Duga S, Angelica Merlini P. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–6.
Gretarsdottir S, Helgason H, Helgadottir A, Sigurdsson A, Thorleifsson G, Magnusdottir A, et al. A splice region variant in LDLR lowers Non-high density lipoprotein cholesterol and protects against coronary artery disease. PLoS Genet. 2015;11(9), e1005379. doi:10.1371/journal.pgen.1005379.
Cholesterol Treatment Trialists’ (CTT) Collaboration. The effects of lowering LDL cholesterol with statin therapy in people at low risk for vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90.
Mega JL, Stitzel NO, Smith JG, et al. Genetic risk, coronary heart disese evetns, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–71.
Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–8.
Schunkert H, Samani N. Statin treatment: can genetics sharpen the focus? Lancet. 2015;385:2227–9.
Sattar N, Preiss D, Murray HK, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomized statin trials. Lancet. 2010;375:735–42.
Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64.
Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks for statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–71.
Swerdlow D, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes and bodyweight: evidence from genetic analysis and randomized trials. Lancet. 2015;385:351–61.
Cohen JC, Boerwinkle E, Mosley Jr TH, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Kent ST, Rosenson RS, Avery CL, et al. PCSK9 loss-of-function variants, low-density lipoprotein cholesterol, and risk of coronary heart disease and stroke: data from the REGARDS Study and CHARGE Consortium. Circulation 2015;132:A9793.
Robinson JG, Farnier M, Krempf M, Bereron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, for the Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) investigators, et al. Efficacy and safety of evolocumbab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9.
Davis Jr HR, Zhu LJ, Hoos LM, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–92.
Altmann SW, Davis Jr HR, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol aborption. Science. 2004;303:1201–4.
Stitziel NO, Won H-H, Morrison AC, The Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med. 2014;371:2072–82.
Ference BA, Majeed F, Penmetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both. A 2 × 2 factorial mendelian randomization study. J Am Coll Cardiol. 2015;65:1552–61.
Cannon CP, Blazing MA, Giugliano RP, IMPROVE-IT Investigators, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Hegele RA, Guy J, Ban MR, Wang J. NPC1L1 haployte is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis. 2005;4:16.
Rosenson RS, Brewer Jr HB, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19.
Glomset JA. The plasma lecithin: cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–67.
Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80.
Rosenson RS, Brewer Jr HB, Ansell B, et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation. 2013;128:1256–67.
Rosenson RS, Brewer HB Jr., Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol 2016;13:48–60.
Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:2525–40.
Barter PJ, Rye K-A. Cholesteryl ester transfer proten inhibition as a strategy to reduce cardiovascular risk. J Lipid Res. 2012;53:1755–66.
Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299:2777–88.
Ridker PM, Pare G, Parker AN, Zee RYL, Miletich JP, Chasman DI. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genomewide analysis among 18,245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2:26–33.
Barter PJ, Caulfield M, Eriksson M, ILLUMINATE Investigators, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22.
Schwartz GG, Olsson AG, Abt M, dal-OUTCOMES Investigators, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Lilly press release October 12, 2015. Visit:http://www.prnewswire.com/news-releases/lilly-to-discontinue-development-of-evacetrapib-for-high-risk-atherosclerotic-cardiovascular-disease-300157604.html.
Cannon CP, Shah S, Dansky HM, Determining the Efficacy and Tolerability Investigators, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–15.
Hovingh GK, Kastelein JJ, van Deventer SJ, et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015;386:452–60.
Rosenson RS, Brewer Jr HB. New challenges for HDL-modifying therapies as a strategy to lower cardiovascular disease events in statin-treated patients. Cardiovasc Drugs Ther. 2015;29:1–3.
Tardif JC, Rhéaume E, Lemieux Perreault LP, et al. Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib. Circ Cardiovasc Genet. 2015;8:372–82.
The TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–3.
Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, et al. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41.
Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–47.
Gaudet D, Brisson D, Tremblay K, Alexander VJ, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–6.
C-Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC). Association between C reactive protein and coronary heart disease: menedelian randomization analysis based on individual participant data. BMJ. 2011;342.
Elliott P, Chambers JC, Zhang W, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48.
Keavney B, Danesh J, Parish S, et al. Fibrinogen and coronary heart disease: test of causality by “Mendelian randomization”. Int J Epidemiol. 2006;35:935–43.
Li L, He M, Zhou L, Miao X, et al. A solute carrier family 22 member 3 variant rs3088442 G → A associated with coronary heart disease inhibits lipopolysaccharide-induced inflammatory response. J Biol Chem. 2015;290:5328–40.
Amerziane N, Beillat T, Verpillat P, et al. Association of the Toll-like recpeotor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol. 2003;23:e61–4.
Ballistreri CR, Candore G, Colonna-Romano G, et al. Roll of Toll-like receptor 4 in acute myocardial infarction and longevity. JAMA. 2004;292:2339–40.
Kolek MJ, Carlquist JF, Muhlestein JB, et al. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am Heart J. 2004;148:1034–40.
Guven M, Ismailoglu Z, Batar B, et al. The effect of genetic polymorphisms of TLR2 and TLR4 in Turkish patients with coronary artery disease. Gene. 2015;558:99–102.
Alarcon G-V, Martinez J A, Villarreal-Molina T, et al. Interleukin-17A gene haplotypes are associated with risk of premature coronary artery disease in Mexican patients from the Genetics of Atherosclerotic Disease (GEA) study. PLoS One 2015.
Biscetti F, Porreca CF, Bertucci F, et al. TNFRSF11B gene polymorphisms increased risk of peripheral arterial occlusive disease and critical limb ischemia in patient with type 2 diabetes. Acta Diabetol. 2014;51:1025–32.
Rosenson RS. Myocardial injury, the acute phase response and lipoprotein metabolism. J Am Coll Cardiol. 1993;22:933–40.
Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications of disease. FASEB J. 2001;15:43–58.
Reich D, Patterson N, Ramesh V, the Health, the Health, Aging and Body Composition (Health ABC) Study, et al. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am J Hum Genet. 2007;80:716–26.
Stephens OW, Zhang Q, Qu P, et al. An intermediate-risk multiple myeloma subgroup is defined by sIL6r levels synergistically increase with incidence of SNP rs2228145 and 1q21 amplification. Blood. 2012;119:503–12.
IL6R Genetic Consortium and Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–13.
The Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisaton analysis. Lancet. 2012;379:1214–24.
Melton L. Coobs, A, Actemra poised to launch IL-6 inhibitors. Nat Biotechnol. 2008;26:957–9.
Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23:49–59.
Rosenson RS, Gelb MH. Secretory phospholipase A2: a multifaceted family of proatherogenic enzymes. Curr Cardiol Rep. 2009;11:445–51.
Rosenson RS. Phospholipase A2 inhibition and atherosclerotic vascular disease: Prospects for targeting secretory and lipoprotein associated phospholipase A2 enzymes. Curr Opin Lipidol. 2010;21:473–80.
Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012;33:2899–909.
Ait-Oufella H, Herbin O, Lahoute C, et al. Group X secreted phospholipase A2 limits the development of atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2013;33:466–73.
Ishizaki J, Hanasaki K, Higashimo K, et al. Molecular cloning of pancreatic group I phospholipase A2 receptor. J Biol Chem. 1994;269:5897–904.
Lambeau G, Ancian P, Barhanin J, Lazdunski M. Cloning and expression of a membrane receptor for secretory phospholipases A2. J Biol Chem. 1994;269:1575–8.
Mishina H, Watanabe K, Tamaru S, et al. Lack of phospholipase A2 receptor increases susceptibility to cardiac rupture after myocardial infarction. Circ Res. 2014;114:493–504.
Nijmer R, Lagrand W, Baidoshvili A, et al. Secretory type II phospholipase A2 binds to ischemic myocardium during myocardial infarction in humans. Cardiovasc Res. 2002;53:138–46.
Rosenson RS, Fraser H, Hislop H, Trias J. Varespladib methyl in cardiovascular disease. Exp Opin Invest Drugs. 2010;10:1245–55.
Rosenson RS, Elliot M, Stasiv Y, Hislop C. Effects of varespladib on biomarkers and major cardiovascular events in acute coronary syndrome patients. J Am Coll Cardiol. 2010;56:1079–88.
Rifai N, Ridker PM. High-sensitivity C-reactive protein: a novel and promising marker of coronary heart disease. Clin Chem. 2011;47:403–11.
Rosenson RS, Fraser H, Goulder MA, Hislop C. Anti-inflammatory effects of verespladib methyl in diabetic patients with acute coronary syndrome. Cardiovasc Drugs Ther. 2011;25:539–44.
Nicholls SJ, Cavender MA, Kastelein JJ, et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. Cardiovasc Drugs Ther. 2012;26:71–5.
Nicholls SJ, Kastelein JJ, Schwartz GG, for the VISTA-16 investigators, et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–62.
Holmes MV, Simon T, Exeter HJ, et al. Secretory phospholipase A(2)-IIA and cardiovascular disease: a mendelian randomization study. J Am Coll Cardiol. 2013;9(62):1966–76.
Holmes MV, Exeter HJ, Folkersen L, et al. Novel genetic approach to investigate the role of plasma secretory phospholipase A2 (sPLA2)-V isoenzyme in coronary heart disease: modified Mendelian randomization analysis using PLA2G5 expression levels. Circ Cardiovasc Genet. 2014;7:144–50.
Guardiola M, Exeter HJ, Perret C, et al. PLA2G10, gene variants, sPLA2 activity, and coronary heart disease risk. Circ Cardiovasc Genet. 2015;8:356–62.
Rosenson RS, Hurt-Camejo E. Letter to the Editor: limits of mendelian randomization analyses in selection of secretory phospholipase A2-IIA as a valid therapeutic target for cardiovascular disease prevention. J Am Coll Cardiol. 2014;63:942–3.
Grallert H, Dupuis J, Bis JC, et al. Eight genetic loci associated with variation in lipoprotein-associated phospholipase A2 mass and activity and coronary heart disease: meta-analysis of genome-wide association studies from five community-based studies. Eur Heart J. 2012;33:238–51.
Thompson A, Gao P, Orfei L, et al. Lp-PLA(2) studies collaboration. Lipoprotein-associated phospholipase a(2) and risk of coronary disease, stroke, and mortality. Lancet. 2010;375:1536–44.
O’Donoghue ML, Braunwald E, White HD, SOLID-TIMI 52 Investigators, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–15.
Rosenson RS, Stafforini DM. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J Lipid Res. 2012;53:1767–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenson, R.S., Koenig, W. Mendelian Randomization Analyses for Selection of Therapeutic Targets for Cardiovascular Disease Prevention: a Note of Circumspection. Cardiovasc Drugs Ther 30, 65–74 (2016). https://doi.org/10.1007/s10557-016-6642-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6642-9