Abstract
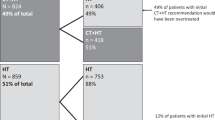
Risk stratification based on results provided by a 21-gene assay (Oncotype DX®) in early stage breast cancer can help optimize hormone therapy (HT) and/or chemotherapy (CT) decisions. We performed a systematic review and meta-analysis of decision impact (DI) and net change in CT use before and after assay results, both in the whole studies’ population and by recurrence risk score (RS) strata. A systematic search of studies with prospective data collection reported physician’s decision on treatment allocation in early stage node-negative breast cancer was performed. DI reflects the proportion of patients whose management was changed, and net change focuses on CT change. A random-effects model is reported. Fifteen studies (N = 2229) met our inclusion criteria: 50.09, 37.35, and 13.38 % of patients with low, intermediate, and high RS. Treatment decision changed in 29.5 % (95 % CI 26.29–32.86). Net reduction of CT use was 12 % (8–17 %). It was 16 % (12.00–19.00) in the low RS group, 0 % (−3.00 to 3.00) in the intermediate RS group, and increased by 2 % (−1.00 to 3.00) in the high RS group. Use of a 21-gene assay showed a significant impact on treatment decisions. From 100 women tested, 30 could have their treatment optimized, and 12 could avoid CT. Its main effects consist of sparing chemotherapy in low risk patients and slightly increasing it in the high risk category. DI could be higher in selected patient populations with greater uncertainty regarding initial treatment decisions.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Ervik M, et al. (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013; http://globocan.iarc.fr. Accessed 01 Jul 2013
Early Breast Cancer Trialists’ Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Hingorani AD, Windt DA, Riley RD et al (2013) Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ 346:e5793
Issa AM, Chaudhari VS, Marchant GE (2015) The value of multigene predictors of clinical outcome in breast cancer: an analysis of the evidence. Expert Rev Mol Diagn 15(2):277–286
Blohmer JU, Rezai M, Kümmel S et al (2013) Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early-stage breast cancer: a cost-effectiveness evaluation in the German setting. J Med Econ 16(1):30–40
Vataire AL, Laas E, Aballea S, Gligorov J, Rouzier R, Chereau E (2012) Cost-effectiveness of a chemotherapy predictive test. Bull Cancer 99(10):907–914
Holt S, Bertelli G, Humphreys I et al (2013) A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the UK. Br J Cancer 108(11):2250–2258
National Institute for Health and Clinical Excellence (2013) NICE diagnostics guidance [DG10]: Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat. http://www.nice.org.uk/guidance/dg10. Accessed July 2014
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
National Heart Lung and Blood Institute, Research Triangle Institute International. Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. 2014; http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after.htm. Accessed July 2014
StatsDirect statistical software. [computer program] (2013). StatsDirect Ltd, England
Bianchini G, Pusztai L, Karn T et al (2013) Proliferation and estrogen signaling can distinguish patients at risk for early versus late relapse among estrogen receptor positive breast cancers. Breast cancer Res 15(5):R86
Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA (2013) Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract 9(4):182–187
Higgins JP, Green S (2008). Cochrane handbook for systematic reviews of interventions. Vol 5: Wiley Online Library, Chichester
Albanell J, Gonzalez A, Ruiz-Borrego M et al (2012) Prospective transGEICAM study of the impact of the 21-gene Recurrence Score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol 23(3):625–631
Bargallo JER, Lara F, Shaw Dulin RJ et al (2012) A study of the impact of the 21-gene breast cancer assay on the use of adjuvant chemotherapy in Women with breast cancer in a Mexican Public Hospital. ESMO, Vienna
Cheung PS, Tong AC, Leung RC, Kwan WH, Yau TC (2014) Initial experience with the Oncotype DX assay in decision-making for adjuvant therapy of early oestrogen receptor-positive breast cancer in Hong Kong. Hong Kong Med J 20:401
Davidson JA, Cromwell I, Ellard SL et al (2013) A prospective clinical utility and pharmacoeconomic study of the impact of the 21-gene Recurrence Score(R) assay in oestrogen receptor positive node negative breast cancer. Eur J Cancer 49(11):2469–2475
de Boer RH, Baker C, Speakman D, Chao CY, Yoshizawa C, Mann GB (2013) The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer. Med J Aust 199(3):205–208
Eiermann W, Rezai M, Kummel S et al (2013) The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol 24(3):618–624
Gerson Cwilich R, Alban de la Torre LF, Villalobos Prieto A, Serrano Olvera JA (2012) Clinicopathological features, prognosis and influence in the adjuvant treatment of the risk recurrence groups determined by the 21 gene expression profile, Oncotype Dx(R), in early breast cancer. Gac med de Mex 148(2):117–124
Gligorov J, Pivot XB, Naman HL et al (2012) Prospective study of the impact of using the 21-gene recurrence score assay on clinical decision making in women with estrogen receptor-positive, HER2-negative, early-stage breast cancer in France. J Clin Oncol 30(15):568
Henry LR, Stojadinovic A, Swain SM, Prindiville S, Cordes R, Soballe PW (2009) The influence of a gene expression profile on breast cancer decisions. J Surg Oncol 99(6):319–323
Klang SH, Hammerman A, Liebermann N, Efrat N, Doberne J, Hornberger J (2010) Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health 13(4):381–387
Levine M, Julian J, Cochrane B (2014) The OCOG Oncotype DX® Field Evaluation Study. ASCO, Chicago
Lo SS, Mumby PB, Norton J et al (2010) Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 28(10):1671–1676
Oratz R, Paul D, Cohn AL, Sedlacek SM (2007) Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract 3(4):182–186
Yamauchi H, Nakagawa C, Takei H et al (2014) Prospective study of the effect of the 21-gene assay on adjuvant clinical decision-making in Japanese women with estrogen receptor-positive, node-negative, and node-positive breast cancer. Clin Breast Cancer 14(3):191–197
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
Hornberger J, Chien R. Meta-analysis of the Decision Impact of the 21-Gene Breast Cancer Recurrence Score® in Clinical Practice. St Gallen International Breast Cancer Conference; 2011; St Gallen, Switzerland
Martinez-Ferez I (2014) Marquez-Pel aez S, Romero-Tabares A, Beltr an-Calvo C. Prognostic genomic tests in breast cancer: clinical utility, efficiency and impact on clinical decision making. Eur J Cancer 50:S200
Albanell J, Gligorov J H, S., Blohmer J, Eiermann W, Svedmann C. Meta-analysis of Prospective European Studies Assessing the Impact of Using the 21-Gene Recurrence Score Assay on Clinical Decision Making in Women with ER-positive, HER2-negative Early Stage Breast Cancer. European Society of Medical Oncology; 2012; Vienna, Austria
Burke E, Trodden D, Plun-Favreau J, Sing AP (2014) The 21-gene breast cancer assay: a roadmap of clinical evidence. Eur J Cancer 50:S206
Hormone therapy with or without combination chemotherapy in treating women who have undergone surgery for node-negative breast cancer (The TAILORx Trial)—NCT00310180. ClinicalTrials.gov 2014; http://clinicaltrials.gov/show/NCT00310180. Accessed 08/08, 2014
van de Vijver MJ, He YD, van ‘t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. New Engl J Med. 347(25):1999–2009
Bartlett JMS, Thomas J, Ross DT et al (2010) Mammostrat (R) as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy. Breast Cancer Res 12(4):R47
Simon RM, Paik S, Hayes DF (2009) Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 101(21):1446–1452
Acknowledgments
This study was funded by an independent and unrestricted grant from Genomic Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are independent of GH; GH had no involvement in study design, analysis, and reporting of the results.
Rights and permissions
About this article
Cite this article
Augustovski, F., Soto, N., Caporale, J. et al. Decision-making impact on adjuvant chemotherapy allocation in early node-negative breast cancer with a 21-gene assay: systematic review and meta-analysis. Breast Cancer Res Treat 152, 611–625 (2015). https://doi.org/10.1007/s10549-015-3483-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3483-3