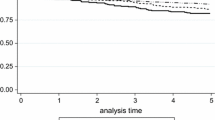
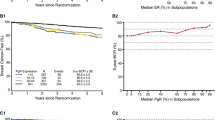
Abstract
The progesterone receptor (PR) has been increasingly well described as an important mediator of the pathogenesis and progression of breast cancer. The aim of this study was to assess the role of PR status as a prognostic factor in addition to other well-established prognostic factors. Data from five independent German breast cancer centers were pooled. A total of 7,965 breast cancer patients were included for whom information about their PR status was known, as well as other patient and tumor characteristics commonly used as prognostic factors. Cox proportional hazards models were built to compare the predictive value of PR status in addition to age at diagnosis, tumor size, nodal status, grading, and estrogen receptor (ER) status. PR status significantly increased the accuracy of prognostic predictions with regard to overall survival, distant disease-free survival, and local recurrence-free survival. There were differences with regard to its prognostic value relative to subgroups such as nodal status, ER status, and grading. The prognostic value of PR status was greatest in patients with a positive nodal status, negative ER status, and low grading. The PR-status adds prognostic value in addition to ER status and should not be omitted from clinical routine testing. The significantly greater prognostic value in node-positive and high-grade tumors suggests a greater role in the progression of advanced and aggressive tumors.




Similar content being viewed by others
References
Eisemann N, Waldmann A, Katalinic A (2013) Epidemiology of Breast Cancer - Current Figures and Trends. Geburtsh Frauenheilk 73(2):130–135. doi:10.1055/s-0032-1328075
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. doi:10.1093/annonc/mdt303
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM (2003) Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21(10):1973–1979. doi:10.1200/JCO.2003.09.099
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 29(32):4273–4278. doi:10.1200/JCO.2010.31.2835
Obr AE, Edwards DP (2012) The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol 357(1–2):4–17. doi:10.1016/j.mce.2011.10.030
Ismail PM, Li J, DeMayo FJ, O’Malley BW, Lydon JP (2002) A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489
Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S (2002) Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol 80:137–148
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A, Glimcher L, Pasparakis M, Khokha R, Ormandy CJ, Widschwendter M, Schett G, Penninger JM (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468(7320):98–102. doi:10.1038/nature09387
Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R (2010) Progesterone induces adult mammary stem cell expansion. Nature 465(7299):803–807. doi:10.1038/nature09091
Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE (2010) Control of mammary stem cell function by steroid hormone signalling. Nature 465(7299):798–802. doi:10.1038/nature09027
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C (2010) Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107(7):2989–2994. doi:10.1073/pnas.0915148107
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50
Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, Allred DC, McCarthy M, Ullrich RL (2002) Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J 16:881–883
Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J (2003) Hormone dependence in premalignant mammary progression. Cancer Res 63:1067–1072
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28(3):509–518. doi:10.1200/JCO.2009.23.1274
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784. doi:10.1016/S0140-6736(11)60993-8
Luftner D, Lux MP, Maass N, Schutz F, Schwidde I, Fasching PA, Fehm T, Janni W, Kummel S, Kolberg HC (2012) Advances in Breast Cancer - Looking Back over the Year. Geburtsh Frauenheilk 72(12):1117–1129. doi:10.1055/s-0032-1328084
Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M (2005) Retrospective Analysis of Time to Recurrence in the ATAC Trial According to Hormone Receptor Status: an Hypothesis-Generating Study. J Clin Oncol 23:7512–7517
Hoefnagel LD, Moelans CB, Meijer SL, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van Gils CH, van der Wall E, van Diest PJ (2012) Prognostic value of estrogen receptor alpha and progesterone receptor conversion in distant breast cancer metastases. Cancer. doi:10.1002/cncr.27518
Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, van der Valk P, van der Groep P, de Vries EG, van der Wall E, van Diest PJ (2010) Receptor conversion in distant breast cancer metastases. Breast Cancer Res 12(5):R75. doi:10.1186/bcr2645
Harrell FE Jr, Lee KL, Pollock BG (1988) Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 80(15):1198–1202
Sauerbrei W, Schumacher M (1992) A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 11(16):2093–2109
Simon R, Altman DG (1994) Statistical aspects of prognostic factor studies in oncology. Br J Cancer 69(6):979–985
Grambsch PM, Therneau TM (1994) Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 81(3):515–526
Chambless LE, Diao G (2006) Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med 25(20):3474–3486. doi:10.1002/sim.2299
Gronnesby JK, Borgan O (1996) A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal 2(4):315–328
Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, Nielsen TO (2010) Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 119(1):53–61. doi:10.1007/s10549-009-0318-0
Yang LH, Tseng HS, Lin C, Chen LS, Chen ST, Kuo SJ, Chen DR (2012) Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer 15(3):288–295. doi:10.4048/jbc.2012.15.3.288
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO (2007) Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol 25(30):4772–4778. doi:10.1200/JCO.2007.12.2747
Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, Montagna E, Iorfida M, Mazza M, Balduzzi A, Veronesi P, Luini A, Intra M, Goldhirsch A, Colleoni M (2012) Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol. doi:10.1093/annonc/mds430
Rosen JM (2003) Hormone receptor patterning plays a critical role in normal lobuloalveolar development and breast cancer progression. Breast Dis 18:3–9
Brisken C, Rajaram RD (2006) Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia 11(3–4):239–248. doi:10.1007/s10911-006-9026-0
Brisken C (2002) Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia 7(1):39–48. doi:10.1023/A:1015718406329
Anderson E (2002) The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4(5):197–201
Shoker BS, Jarvis C, Clarke RB, Anderson E, Munro C, Davies MP, Sibson DR, Sloane JP (2000) Abnormal regulation of the oestrogen receptor in benign breast lesions. J Clin Pathol 53(10):778–783
Sutherland RL, Prall OW, Watts CK, Musgrove EA (1998) Estrogen and progestin regulation of cell cycle progression. J Mammary Gland Biol Neoplasia 3(1):63–72. doi:10.1023/A:1018774302092
Skildum A, Faivre E, Lange CA (2005) Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol 19(2):327–339. doi:10.1210/me.2004-0306
Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB (1997) Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol 11(11):1593–1607
Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL (2009) DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology 150(7):3318–3326. doi:10.1210/en.2008-1630
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360(8):790–800. doi:10.1056/NEJMra0801289
Strehl JD, Wachter DL, Fasching PA, Beckmann MW, Hartmann A (2011) Invasive Breast Cancer: recognition of Molecular Subtypes. Breast Care (Basel) 6(4):258–264. doi:10.1159/000331339
Schmidt M, Fasching PA, Beckmann MW, Kolbl H (2012) Biomarkers in Breast Cancer–an Update. Geburtsh Frauenheilk 72(9):819–832. doi:10.1055/s-0032-1315340
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. doi:10.1200/JCO.2009.25.6529
Acknowledgments
This work was partly funded by the ELAN programme of the University Hospital Erlangen.
Conflict of Interest
AH declares a consultant role for Medac, PAF declares a consultant role for Novartis and research funding by Novartis, WJ declares research funding by Pfizer, AstraZeneca and Novartis. All other authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jessica Salmen and Julia Neugebauer have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salmen, J., Neugebauer, J., Fasching, P.A. et al. Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat 148, 143–151 (2014). https://doi.org/10.1007/s10549-014-3130-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3130-4