Abstract
Invasive insects and fungal pathogens have triggered numerous declines in ecologically important tree species in the forests of eastern North America. Although now functionally extinct in many ecosystems, these tree species have been able to persist through sprouting or the presence of a low density of resistant individuals. In this study, we document widespread mortality of another dominant North American hardwood species, redbay (Persea borbonia, Lauraceae), from an exotic disease, laurel wilt. This disease is caused by a nonnative Asian ambrosia beetle, Xyleborus glabratus, and its pathogenic fungal symbiont, Raffaelea lauricola, which were introduced to Georgia, USA in 2003. We conducted tree surveys on an island near the center of introduction from 2004 to 2009 and assessed the trajectory of tree mortality caused by laurel wilt. Additionally, we examined sprouting as a mechanism for persistence and whether changes in community structure occurred after laurel wilt introduction. We observed 98 % death of main stems and widespread mortality of genets (79 %) independent of main stem size. All remaining individuals were symptomatic of laurel wilt. Sprouting does not appear to give redbay the ability to maintain genets and recruit new stems into the forest canopy. We identified a negative interaction between laurel wilt and deer browse on stems and new sprouts, which may accelerate death rates of infected populations. If our results are applicable across redbay’s geographic range, a once abundant tree species may become ecologically extinct from coastal forest ecosystems in the southeastern United States.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive exotic insects and fungal pathogens, acting alone or together as symbionts, have been responsible for the dramatic decline of a number of ecologically important tree species in the forests of eastern North America (Liebhold et al. 1995). The loss of these tree species has led to fundamental changes in the composition, structure and function of the forest communities in which they were found. In the early twentieth century, the American chestnut (Castanea dentata) was eliminated as a dominant canopy species throughout its range by the introduction of the chestnut blight (Cryphonectria parasitica) (Milgroom 2004). Loss of chestnut resulted in the alteration of forest ecosystem processes such as nutrient cycling, decomposition, understory competitive interactions, canopy compositional change, and trophic webs associated with its annual supply of nuts (Loo 2008). Dutch elm disease, caused by beetle-transmitted fungal species (Ophiostoma ulmi, O. novo-ulmi and O. himal-ulmi), decimated populations of American elm (Ulmus americana), an important riparian species (Harrington et al. 2001; Cheng et al. 1997). Dogwood anthracnose fungus (Discula destructiva) caused major declines in flowering dogwood (Cornus florida) populations—a major contributor to songbird food and calcium cycling (Hiers and Evans 1997). The recent spread of the hemlock wooly adelgid (Adelges tsugae) from New England southward eliminated stands of eastern hemlock (Tsuga canadensis) and Carolina hemlock (Tsuga caroliniana), causing local losses in associated animal species (Tingley et al. 2002; Ellison et al. 2005), regional homogenization of plant and animal diversity (Ellison et al. 2005), changes in soil ecosystem dynamics (Jenkins et al. 1999), and altered hydrological regimes (Siderhurst et al. 2010). Despite the rapid declines in each of these species, some populations persist through understory sprouting and/or the maintenance of a low density of resistant individuals (Loo 2008) and at the edges of their ranges.
We document the decline of yet another common North American hardwood species, redbay (Persea borbonia, Lauraceae), from an exotic, invasive disease, laurel wilt. This disease emerged from the introduction of a nonnative Asian ambrosia beetle, Xyleborus glabratus, and its pathogenic symbiont— the fungus, Raffaelea lauricola. Following the introduction of the Asian ambrosia beetle at port Wentworth, GA in 2002, widespread mortality of redbay was first observed in South Carolina and Georgia starting in 2004 (Fraedrich et al. 2007, 2008; Cameron et al. 2008; Shields et al. 2011). By 2009, laurel wilt had spread throughout coastal Georgia and into Florida (Fraedrich et al. 2007). Redbay is an important component of both the canopy and subcanopy strata within coastal plain forests and barrier island maritime forests of southeastern United States (Bellis 1995) and are a dominant species on tree islands in the Everglades (Sklar and van der Valk 2002). Its high abundance (Bratton and Miller 1994) and nutritional fruits make redbay an important source of habitat and food for animal species (Brendemuehl 1990). Some fauna, such as the palamedes swallowtail butterfly (Papilio palamedes) and the leaf miner (Phyllocnistis subpersea) are dependent on redbay as a specific host food plant (Lederhouse et al. 1992; Scriber and Margraf 2000; Davis and Wagner 2011).
Although some studies have provided an initial assessment of the ecological impacts of laurel wilt (e.g., see Fraedrich et al. 2008; Shields et al. 2011), no study has documented the stand-level trajectory of redbay mortality since laurel wilt was first introduced or has examined whether basal sprouting has allowed for the persistence of genetic individuals (genets) within populations. We accomplished both of these objectives and have additionally examined whether the decline of redbay has allowed for the recruitment of new species and/or promoted the regeneration of existing species within the maritime forest community.
Methods
Study site
Our study was conducted on St. Catherine’s Island, a Georgia barrier island located 50 km south of the 2002 initial introduction of laurel wilt. The first symptoms of laurel wilt appeared on St Catherine’s Island in 2004 (Cameron et al. 2008). The forests on St. Catherine’s Island are characterized by diverse communities of coastal maritime species with similar physiography and biota to other barrier islands on the southern Georgia coast (Bratton and Miller 1994; Coile and Jones 1988). Similar to other maritime forests and forest ecosystems in southeastern United States, the maritime forests on this island have a long history of anthropogenic impacts (Thomas et al. 1978). Much of the central portion of the island was converted to cotton fields between 1800 and 1860 (Thomas et al. 1978; Coile and Jones 1988), and used for cattle production in the mid-1900s. Today, the island is managed as an ecological reserve where small remnant stands of primary forest are interspersed throughout a matrix of second growth hardwood and pine forest (Evans and Keen 2013). Prior to the introduction of laurel wilt, redbay was a common canopy and understory species in both primary and second growth stands across the island (Coile and Jones 1988). High white-tailed deer density, occurring in the absence of hunting and predation, has resulted in a distinct browse line across the island (Coile and Jones 1988; Evans and Keen 2013).
Population sampling
We examined the consequences of laurel wilt disease progression on the population structure of red bay across our study area. From 2004 to 2009, we sampled redbay from 1 ha plots located within six forest stands located across the north to south extent of the island. Stands were chosen to represent the variation in species composition and past landuse across the island.
Within each plot, we located and counted each redbay genet (a genet consisted of a main stem and associated basal sprouts—this is the typical condition of redbay). For each genet we recorded either height (if >= 0.5 m but <1.5 m) or dbh (stem diameter in cm at 1.5 m ht) of the main (largest) stem. Seedlings (<0.5 m in ht) were not censused but were noted as being uncommon. We also determined the percent of foliage death of the main stem (0, 1–25, 26–50, 51–75, 76–99, 100) and noted whether or not basal sprouts were present. Plots were sampled using these methods from 2004 to 2007 and again in 2009. During the years we conducted our study, the USDA and the Georgia Forestry Commission conducted regular surveys documenting the spread of R. lauricola and X. glabratus across the barrier islands of Georgia including St Catherines Island (Cameron et al. 2008).
Trajectory of genet death
When a main stem died at a genet location, we continued to track basal sprout survival in subsequent years at that genet location until there was total genet death. We identified three states that characterize the status of genets from the onset of infection through to genet mortality: “state 1”—main stem with leaf senescence of <25 % and onset of epicormic and basal sprouting; “state 2”—main stem with leaf senescence of 25–99 % and continued epicormic and basal sprouting; “state 3a”—death of main stem, with basal sprouting below 1.5 m in height (i.e., below the deer browse line); “state 3b”—death of main stem with basal sprouting above 1.5 m in height (i.e., above the browse line of deer). Genet mortality is characterized by complete death of main stem and basal sprouts.
Sprouting persistence
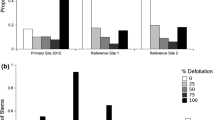
In 2007 and 2009, we determined the height of the tallest basal sprout at each genet location. We observed that the majority of basal sprouts in 2007 exhibited signs of deer browse. If the tallest basal sprout was found to be >1.5 m, then it was considered to be out of the deer browse zone. In 2009, we selected three 0.25 ha areas within our six 1 ha sampling plots and quantified deer browse on the basal sprouts at each genet location. Intensity of deer browse on sprouts was visually assessed at each genet location using a 0–3 scale (0—no evidence of browse, 1—1–25 % defoliation, 2—26–50 % defoliation, 3 -> 50 % defoliation and multiple meristems affected).
Community composition
In 2007 and 2009, we recorded the dbh and relative canopy position of all stems of all woody species in each plot that were >= 0.5 m in height. Canopy positions were as follows: canopy (individuals with no overhead tree cover), subcanopy (>2 m ht but beneath canopy trees) and understory (0.5 –2 m). From these data, changes in understory density by species were determined for this two-year period.
Results
Redbay population trends and sprouting persistence
By 2009, only 69 (21 %) of the original 327 genets present in 2004 were still alive (Fig. 1). Of these, only 7 genets with living main stems remained (<2 % of those present in 2004) and only one of these stems had a canopy that was more than 75 % intact (state 1). The other six stems were state 2 individuals, of which five displayed epicormic sprouting—which we observed to be typically associated with a dying stem. In 2009, we observed no fruiting individuals in any stand. Between 2004 and 2006, state 1 genets declined in number as they transitioned into state 2 and then state 3 type genets (Fig. 1). The highest number of dead main stems with sprouts (state 3) was in 2006, 2 years post-invasion. In 2009, there were 62 genets in state 3 and 23 % (14) of these had sprouts that grew above browse line (state 3b). However, there was 78 % mortality in state 3 genets between 2006 and 2009— indicating sprouting does not appear to be a long term mechanism for persistence in a wilt stricken population (Fig. 1). No (non-sprout) saplings (between 0.5 and 1.5 m in ht) were observed in any stand during our study.
The total number and status of live redbay genets sampled from six 1-hectare forest plots on St. Catherine’s Island, Georgia, USA between 2004 and 2009. We identified the following states that characterize the status of genets from the onset of infection through genet mortality: “state 1”—main stem with leaf senescence of <25 % and onset of epicormic and basal sprouting; “state 2”—main stem with leaf senescence of 25–99 % and continued epicormic and basal sprouting; “state 3”—death of main stem, with basal sprouting; “dead”—death of genet
In 2004, there was not an inverse-J size class distribution of live stems. This relative lack of representation in the smaller size classes indicated that a regeneration failure was occurring in the redbay population prior to the laurel wilt epidemic. We observed 98 % death of main stems and widespread mortality of genets (79 %), independent of main stem size (Fig. 2). Regardless of size class, death of main stems resulted in basal sprouting (Fig. 2).
Comparison of redbay size class distributions of different genet states from six 1-hectare forest plots on St. Catherine’s Island. The graph on the left shows the overall decline and change in distribution of live main stems within the population between 2004 and 2009. The graph on the right shows sprouting only individuals (state 3 genets) from 2006 to 2009 as a function of the size of their original (now dead) main stem
Deer browse on redbay sprouts
Sampling of browse intensity on sprouts indicated that 93 % of all basal sprouting displayed medium to high levels of deer browse. In 2009, surviving state 3b genets were from individuals whose main stem sizes were less than 20 cm (Fig. 2).
Change in community composition
Despite wide variation in stand composition, redbay was the most abundant subcanopy tree across all stands prior to laurel wilt invasion—representing 17–54 % of total individuals in this stratum of the forest (Table 1). Redbay was the third most abundant tree in the forest canopy with variation across stands (Table 1; range 1–36 %). Over the course of the 6 years, a major structural change occurred in these stands as laurel wilt almost completely eliminated redbay from the forest subcanopy and canopy. Despite this change, by 2007, no understory regeneration was occurring for any of the major canopy and subcanopy tree species, except Pinus taeda. All hardwood canopy tree species lacked representation in the 0.5–2 m ht size class and this remained unchanged by 2009 (Table 2). The only subcanopy species showing some positive change in density by 2009 were two large shrub species, Morella cerifera and Vaccinium arboreum. However, these species only increased an average of 3.7 stems per hectare across the stands (Table 2).
Discussion
Redbay decline
We document widespread mortality within a redbay population from laurel wilt invasion. Over 6 years (2003–2009), a clear transition between genet mortality states was observed as a result of laurel wilt—after 2 years there was full canopy death of live stems (state 2) and after 4 years the majority of live stems died and genets were manifested as sprouts (state 3). By the sixth year post-invasion, genet mortality occurred throughout the population despite sprouting. Unlike in other tree species affected by invasive pathogens and insects (Liebhold et al. 1995; Runkle 2007), sprouting does not appear to give redbay the ability to maintain genets and recruit new stems back into the forest canopy and subcanopy. With the removal of canopy and subcanopy trees there was a complete absence of new seed production within the population.
Laurel wilt, deer browse and community response
The forest’s vertical structure was fundamentally altered by laurel wilt through the almost complete elimination of subcanopy and canopy redbay trees. However, no hardwood species showed evidence of new recruitment in response to this change. The lack of a current regeneration in any of these species (including redbay in 2004) suggests that there has been a long-term hardwood regeneration failure independent of laurel wilt (Table 2). Indeed, Evans and Keen (2013) observed no recruitment in pignut hickory (Carya glabra) on St. Catherine’s Island. They suggest that deer browse is preventing hardwood sapling recruitment. The 2004 size class distribution of redbay clearly shows the absence of sapling size classes and our data indicate high levels of browse on redbay sprouts. Thus, it appears that deer browse may be promoting redbay mortality in laurel wilt stricken populations. Although some sprouts resulting from main stem death were able to surpass the browse line, sprouting did not guarantee genet survival as genets with sprouts continued to decline from 2007 to 2009, regardless of whether sprouts were above or below the browse line.
Future implications
There are three population sources that could contribute to a redbay population recovery on St. Catherine’s Island: (1) persistent sprouts, (2) seed pool, and (3) dispersed seed from other islands. All three sources of regeneration are presently inhibited by deer and/or laurel wilt. Deer may be exhausting root reserves in this continual sprouting-browsing regime (Cote et al. 2004) and thus accelerating genet death. It is possible that sprouts above the browse line may have increased chances of survival in the future if beetle populations decline due to reduction in live redbay stems (Hanula et al. 2008). Redbay does not maintain persistent seed banks in the soil and most seeds are not viable beyond 2 years (Vankus 2009). With the absence of fruit producing trees by 2009, the population is no longer capable of producing seedlings. Therefore the only means for future redbay genet establishment within these stands will be through vicarious seed dispersal to the island by birds. Howover, there is also no evidence that redbay seedlings are currently able to transition to sapling height (0.5–1.5 m). Moreover, if regional trends of redbay mortality due to laurel wilt are realized (Shields et al. 2011), external seed sources may also vanish as the species disappears from the southeastern landscape.
As redbay approaches ecological extinction across its range, the long-term effects on forest ecosystems remain unknown. Animals that specifically depend upon redbay as a host food plant such as the palamedes butterfly may experience major declines populations (Koch and Smith 2008), although there is no evidence yet to confirm this. We do know from our study on St Catherine’s Island that the loss of redbay fundamentally changes forest structure and this can have many potential consequences: (1) higher light penetration will affect understory thermal regimes and likely influence herbaceous composition and productivity as well as ground-dwelling animals; (2) the dramatic reduction in subcanopy stem density will result in lower vertical complexity of the forest as habitat for nesting birds; and (3) decreased litter input to the forest floor may affect patterns of nutrient cycling over time and influence the composition of soil biota. Given its formerly high relative abundance, the loss of redbay may have far reaching implications for biodiversity in coastal forest ecosystems of the southeastern United States.
References
Bellis VJ (1995) Ecology of maritime forests of the southern Atlantic Coast: a community profile. Biological Report 30. US Department of the Interior, National Biological Service, Washington D.C.
Bratton SP, Miller SG (1994) Historic field systems and the structure of maritime oak forests, Cumberland Island National Seashore, Georgia. Bull Torrey Botan Club 121:1–12
Brendemuehl RH (1990) Persea borbonia (L) Spreng: Redbay. In: Burns RM, Honkala BH (eds) Silvics of North America 2: hardwoods. United States Department of Agriculture, Washington, pp 503–506
Cameron RS, Bates C, Johnson J (2008) Distribution and spread of laurel wilt disease in Georgia, 2006–2008 survey and field observations. Georgia Forestry Commission report. 28 p. http://www.fs.fed.us/r8/foresthealth/laurelwilt/resources/pubs/georgia_laurel_wilt_report_2006-08.pdf
Cheng ZM, Shi NQ, Herman DE, Capps TK (1997) Building in resistance to Dutch elm disease. J For 95:24–27
Coile NC, Jones SB Jr (1988) Checklist of the vascular flora of St. Catherine’s Island, Georgia. Am Mus Novit 2920:1–14
Cote SD, Rooney TP, Tremblay J, Dussault C, Waller DM (2004) Ecological impacts of deer over abundance. An Rev Ecol Evol Syst 35:113–147
Davis DR, Wagner DL (2011) Biology and systematics of the New World Phyllocnistis Zeller leafminers of the avocado genus Persea (Lepidoptera, Gracillariidae). ZooKeys 97:39–73
Ellison AM, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM et al (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486
Evans JP, Keen E (2013) Regeneration failure in a remnant stand of pignut hickory (Carya glabra) on a protected barrier island in Georgia, USA. Nat Areas J 33:171–176
Fraedrich SW, Harrington TC, Rabaglia RJ (2007) Laurel Wilt: a new and devastating disease of redbay caused by a fungal symbiont of the exotic redbay ambrosia beetle. Newslett Mich Entomol Soc 52:14–15
Fraedrich SW, Harrington TC, Rabaglia RJ, Mayfield AE III, Hanula JL, Eickwort JM, Miller DR (2008) A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the Southern United States. Plant Dis 92:215–224
Hanula JL, Mayfield AE, Fraedrich SW, Rabaglia RJ (2008) Biology and host associations of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae), exotic vector of laurel wilt killing redbay trees in the southeastern United States. J Econ Entomol 101:1276–1286
Harrington TC, McNew D, Steimel J, Hofstra D, Farrell R (2001) Phylogeny and taxonomy of the Ophiostoma piceae complex and the Dutch elm disease fungi. Mycologia 93:111–136
Hiers JK, Evans JP (1997) Effects of anthracnose on dogwood mortality and forest composition of the Cumberland Plateau (USA). Conserv Biol 11:1430–1435
Jenkins JC, Aber JD, Canham CD (1999) Hemlock woolly edelgid impacts on community structure and N cycling rates in eastern hemlock forests. Can J For Res 29:630–645
Koch FH, Smith WD (2008) Spatio-temporal analysis of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) invasion in eastern US forests. Environ Entomol 37:442–452
Lederhouse RC, Ayres MP, Nitao JK, Scriber JM (1992) Differential use of Lauraceous hosts by swallowtail butterflies, Papilio troilus and P. palamedes (Papilionidae). Oikos 63:244–252
Liebhold AM, MacDonald WL, Bergdahl D, Mastro VC (1995) Invasion by exotic forest pests: a threat to forest ecosystems. For Sci Monogr 30:1–49
Loo JA (2008) Ecological impacts of non-indigenous invasive fungi as forest pathogens. Biol Inv 11:81–96
Milgroom MG (2004) Biological control of chestnut blight with hypovirulence: a critical analysis. Ann Rev Pathol 42:311–338
Runkle JR (2007) Impacts of beech bark disease and deer browsing on the old-growth forest. Am Midl Nat 157:241–249
Scriber JM, Margraf N (2000) Suitability of four families of Florida “bay” species for Papilio palamedes and P. glaucus (Papilionidae). J Lepidopt Soc 54:131–136
Shields J, Jose S, Freeman J, Bunyan M et al (2011) Short-term impacts of laurel wilt on Redbay (Persea borbonia [L.] Spreng.) in a mixed evergreen-deciduous forest in northern Florida. J For 109:82–88
Siderhurst LA, Griscom HP, Hudy M, Bortolot ZJ (2010) Changes in light levels and stream temperatures with loss of eastern hemlock (Tsuga canadensis) at a southern Appalachian stream: implications for brook trout. For Ecol Manag 260:1677–1688
Sklar F, van der Valk A (2002) Tree Islands of the everglades. Springer, Berlin, p 541
Thomas DH, Jones GD, Durham RS, Larsen CS (1978) The anthropology of St. Catherine’s Island. I. Natural and cultural history. Anthropol Pap Am Mus Nat Hist 55:155–248
Tingley MW, Orwig DA, Field R, Motzkin G (2002) Avian response to removal of a forest dominant: consequences of hemlock woolly adelgid infestations. J Biogeogr 29:1505–1516
Vankus V (2009) Germplasm conservation of Persea borbonia. Presented paper. Laurel Wilt Coference, Savannah, GA (Feb. 27 2009)
Acknowledgments
We would like to acknowledge the support the following students at Sewanee : The University of the South who contributed to this study over the years: Sydney Bowles, Mary Beth Epps, Scott Hardage, Ryan Holland, Johnson Jeffers, Allison Laney, John Roberts, Katherine Ross, James Shaffer, Jim Voitier, Ellen Yungmeyer, Chris Zachau. Thanks to David Haskell, Bert Harris, Leighton Reid and Keri Bryan for reading earlier drafts of the manuscript. Further thanks goes to Nick Hollingshead and to the St. Catherine’s Island Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Evans, J.P., Scheffers, B.R. & Hess, M. Effect of laurel wilt invasion on redbay populations in a maritime forest community. Biol Invasions 16, 1581–1588 (2014). https://doi.org/10.1007/s10530-013-0592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0592-y