Abstract
Pseudomonas stutzeri, isolated from crude oil-contaminated soil, was used to degrade diesel oil. Of three surfactants, 120 mg rhamnolipids 1−1 significantly increased degradation of diesel oil giving 88% loss after 14 days compared to 54% loss without the surfactant. The system with rhamnolipids was characterised by relatively high particle homogeneity. However, the addition of saponins to diesel oil caused the cells to aggregate (the polydispersity index: 0.542) and the biodegradation of diesel oil was only 46%. The cell yield was 0.22 g l−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surface properties of microorganisms are vital for effective biodegradation of hydrophobic sources of carbon. The initial high hydrophobicity of the surface of bacteria cells favours the interactions between the cell surface and the carbon source (Deziel et al. 1999; Stenstrom 1989) which leads to a faster and more efficient utilisation of hydrocarbons. Other factors favouring the biodegradation rate of hydrophobic carbon sources are microbial surfactants. They increase the dispersion of hydrocarbons in water and enlarge the area of contact with bacteria cells. Moreover, biosurfactants can modify the surface of bacteria cells (Lai et al. 2009). Zhang and Miller (1994) suggested that the bioavailability of octadecane in the presence of rhamnolipids is controlled by dispersion of these compounds in water and bacteria cell hydrophobicity. The presence of biosurfactant increases the cell hydrophobicity of slow degraders, but has no effect on the hydrophobicity of fast degraders. Rhamnolipids adsorbed on the cell surface of bacteria can extract membrane lipopolysacharides leading to an increase in hydrophobicity (Al-Tahhan et al. 2000). However, Obuekwe et al. (2009) have described Williamsia muralis as lacking a hydrophobic character but still having a good ability to degrade hydrocarbons. In this case, mechanisms other than hydrophobic interaction allow hydrocarbon uptake by microorganisms.
The aim of the study was to check the applicability of measurements of sedimentation profile and particle size distribution as an effective tool for observation of changes in the properties of Pseudomonas stutzeri cell surface. Their cell surface adhesion to hydrocarbon was assessed using the microbial adhesion to hydrocarbon (MATH) method described by Rosenberg et al. (1980). The influence of different stress factors on the surface properties of the bacteria cells was assessed and the effect of the presence of surfactants (rhamnolipids, Triton X-100, saponins) on efficiency of diesel oil biodegradation was indicated.
Materials and methods
Chemicals
Hydrocarbon and other fine chemicals employed high grade were purchased from Merck (Germany). Surfactants used were: rhamnolipids (Jeneil Biosurfactant Company, USA, JBR 425; content 25% of rhamnolipids), saponins (Sigma) and Triton X-100.
Bacterial strain and growth conditions
Pseudomonas stutzeri AG 22 (biochemical profile: 04573043222) was identified using ID 32 GN biochemical tests (bio-Merieoux, France) and molecular techniques. It had been isolated from soil contaminated with crude oil. The culture medium used throughout these studies was described previously (Kaczorek et al. 2010).
Biodegradation test
Cultures were grown in Erlenmeyer flasks (500 ml) containing 100 ml culture medium (see Table 3) with 2% (w/v) diesel oil. When used, 120 mg surfactant l−1 was added to the medium. Each experiment was repeated five times to attain the accuracy of ±3.7%. Diesel oil content was determined as described in detail by Kaczorek et al. (2010). The final results were calculated with respect to blank samples (hydrocarbon with a medium, without microorganisms).
Microbial adhesion to hydrocarbons
Microbial surface hydrophobicity was assessed with the MATH method described by Rosenberg et al. (1980). The culture was grown on different sources of carbon: hexane, hexadecane, tridecane, diesel oil, glucose, cumene, t-butylbenzene, and three surfactants (rhamnolipids, saponins and Triton X-100) at 120 mg l−1 used alone or in a mixture with diesel oil. Each experiment was repeated three times to attain the accuracy of ±1.2%.
Sedimentation profile evaluation
The sedimentation profile was measured on the K-100 tensiometer (Krüss, Germany) by a suspended probe to directly measure the mass sediment versus time. After 7 days, cells in the late growth phase were centrifuged and the sedimentation profile was determined as described by Kaczorek et al. (2011).
Particle size distribution
Particle size distribution of the tested systems was determined using a Zetasizer Nano ZS apparatus (Malvern Instruments Ltd., UK) applying the non-invasive back light scattering method. On the basis of particle size distribution, the polydispersity index (PdI) was calculated.
Results and discussion
The effect of carbon source on bacterial adhesion to the hydrocarbon used
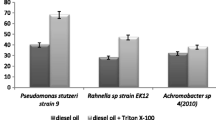
Bacterial adhesion to hydrocarbon depends on the type of carbon source used. During growth on hexadecane the cell surface had hydrophilic properties comparable to those of the parent strain (Table 1). The highest cell surface hydrophobicity (CSH) was noted when the bacterial strain had contact with cumene. Moreover, the effect of surfactants and their concentration was studied and only the effect of Triton X-100 was irrespective of its concentration (Fig. 1a). Different cell surface properties of the tested strain were observed in the systems made of a surfactant and diesel oil. These changes were particularly pronounced in the diesel oil-Triton X-100 system (Fig. 1b), which indicated that surfactants can interact with the microbial cell surface. Moreover, modification of the cell surface was dependent on the time of contact with the carbon source during the experiments (Table 2). Neu (2005) and Zeng et al. (2005) described that contact with surfactants can cause changes in CSH. However, Zhong et al. (2008) have demonstrated that cell surface hydrophobicity is a consequence of surfactant adsorption and that changes in the cell surface depend on the surfactant structure and cell type.
Bacterial adhesion to hydrocarbon determined for Pseudomonas stutzeri AG 22 after 7 days in a surfactant system (a) and surfactant-diesel oil system (b), for different surfactant (Triton X-100, rhamnolipids, saponins) concentrations (g l−1; 6, 30, 60, 120, 240, 360). Cells were centrifuged at 8,000×g for 5 min and washed twice to remove residual hydrocarbons with a PUM buffer (g l−1; 19.7 K2HPO4, 7.26 KH2PO4, 1.8 urea and 0.2 MgSO4·7H2O. Results have absolute (100%) quantitative value
Diesel oil biodegradation
Pseudomonas stutzeri degraded diesel oil (see Table 3) and surfactants increased its efficiency. From among the surfactants used, rhamnolipids were the best for the acceleration of diesel oil degradation. After 14 days, 88% of diesel oil was successfully removed from the system. In the presence of saponins, the biodegradation of diesel oil was comparable to that in the system without a surfactant, though in the diesel oil/saponins system the cell surface was more hydrophilic than that in the system with diesel oil only.
Results of our earlier work have shown that saponins could be good surfactants for hydrocarbon biodegradation in the presence of the Pseudomonas alcaligenes bacteria strain (Kaczorek et al. 2011). CSH of P. alcaligenes was similar to that of P. stutzeri AG 22, which indicated that a mechanism, different than modification of the cell surface, determined the uptake of diesel oil biodegradation. The efficiency of cell surface modification was dependent on the duration of biodegradation. Longer contact time between diesel oil, surfactant and bacteria facilitated surfactant incorporation into the cell membrane and favoured increase in cell hydrophobicity.
Sedimentation profile and particle size distribution
Measurements of sedimentation profile and particle size distribution were tested for their use in evaluating bacteria cell surface modifications. The highest sedimentation of biomass amongst glucose, diesel oil and hexadecane was when glucose was metabolised by P. stutzeri (Fig. 2a). The polydispersity index (PdI), as well as particle size distribution (Fig. 3a) indicated that this system is not homogenous and contained large particles. The sedimentation profiles, PdIs and particle size distribution determined for diesel oil and hexadecane were comparable. The use of surfactants resulted in the formation of cell aggregates, especially when natural surfactants (rhamnolipids and saponins) were used (Fig. 3b). For these surfactants, a high PdI was obtained and the values of the sediment mass were comparable as a result of a higher rate of sedimentation of large particles (Fig. 2b).
Weight of sediment bacterial cells (Pseudomonas stutzeri AG 22) accumulated per 100 ml with time. Bacteria strain was inoculated with different sources of carbon: glucose, diesel oil, hexadecane (a); Triton X-100, rhamnolipids or saponins (b); Triton X-100 + DO, rhamnolipids + DO, saponins + DO (c); surfactants in concentration 120 mg l−1; DO—diesel oil. After 7 days cells were centrifuged at 8,000×g for 5 min and washed twice with PUM buffer (g l−1; 19.7 K2HPO4, 7.26 KH2PO4, 1.8 urea and 0.2 MgSO4·7H2O). 0.1 g of bacterial cells were then suspended in 75 ml of PUM buffer and mixed 120 s directly before measurement
Particle size distribution (PdI) during Pseudomonas stutzeri AG22 growth on a different carbon sources: glucose—PdI: 0.481, diesel oil—PdI: 0.145, hexadecane—PdI: 0.152 (a); surfactants in concentration 120 mg l−1: Triton × 100—PdI: 0.061, rhamnolipids—PdI: 0.421, saponines—PdI: 0.386 (b); Triton X-100 + DO—PdI: 0.285, rhamnolipids + DO—PdI: 0.183, saponines + DO—PdI: 0.542 (c); DO—diesel oil, hexadecane and glucose were added in a concentration of 2% (w/v)
With Triton X-100, the smallest sedimentation mass and particle size were observed. Different values of sediment mass were determined when surfactants were introduced to the systems with diesel oil (Fig. 2c), which meant different types of cell surface modification. Because of the presence of saponins and Triton X-100 the systems’ homogeneity was reduced though they were characterised by a high polydispersity index (Fig. 3c).
However, a different situation was observed in the presence of rhamnolipids. The polydispersity index was smaller in the rhamnolipids/diesel oil system than in the rhamnolipids system. Introduction of rhamnolipids to diesel oil resulted in increasing the homogeneity of the system. Generally, for almost all systems a higher gradient of sedimentation profile resulted in higher values of sediment mass.
A number of methods have been developed for determination of cell surface properties. However, a common problem is the poor correlation between the same parameters determined by different methods (Mozes and Rouxhet 1987). The methods that have been proposed hitherto for determination of the cell surface hydrophobicity either measure overall or localised cell surface and are characterised by sensitivities varying widely (Kjelleberg and Hermansson 1984). Relative hydrophobicity of a bacterial cell can be determined by using for example, microbial adhesion to hydrocarbons (MATH), salting out, hydrophobic interaction chromatography (HIC), adhesion to a nitrocellulose filter or water contact angle measurements. MATH is a simple and rapid method for determination of cell adhesion to hydrocarbon while, according to van Loosdrecht et al. (1987), the contact angle should be recommended as as good measurement of bacterial hydrophobicity. Results obtained using the hydrophobic interaction chromatography (HIC) assay, however, differ completely from those obtained from the MATH assay (Gannon et al. 1991). In our opinion, there is no universal method for hydrophobicity determination and moreover results differ considerably.
Conclusion
Measurements of sedimentation profile and particle size distribution provide information on changes in the bacteria cell surface properties of taking place in the process of diesel oil biodegradation. From among the tested surfactants, rhamnolipids have been found most effective in increasing the rate of diesel oil biodegradation. In general, the efficiency of diesel oil biodegradation (rate of removal of hydrophobic sources of carbon from the environment) is determined by the homogeneity of the system after introduction of surfactants. The type of modification of surface cells of P. stutzeri AG 22 strain is found to depend on the type and concentration of surfactants present.
References
Al-Tahhan RA, Sandrin TS, Bodour AA, Maier RM (2000) Rhamnolipids-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl Environ Microbiol 66:3262–3268
Deziel E, Comeau Y, Villemur R (1999) Two-phase bioreactors for enhanced degradation of hydrophobic/toxic compounds. Biodegradation 10:219–233
Gannon JT, Manilal VB, Alexander M (1991) Relationship between cell surface properties and transport of bacteria through soil. Appl Environ Microbiol 57:190–193
Kaczorek E, Urbanowicz M, Olszanowski A (2010) The influence of surfactants on cell surface properties of Aeromonas hydrophila during diesel oil biodegradation. Colloids Surf B 81:363–368
Kaczorek E, Moszyńska S, Olszanowski A (2011) Modification of cell surface properties of Pseudomonas alcaligenes S22 during hydrocarbon biodegradation. Biodegradation 22:359–366
Kjelleberg S, Hermansson M (1984) Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol 48:497–503
Lai Ch-Ch, Huang Y-Ch, Wei Y-H, Chang J-S (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167:609–614
Mozes N, Rouxhet PG (1987) Methods for measuring hydrophobicity of microorganisms. J Microbiol Methods 6:99–112
Neu TR (2005) Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Mol Biol R 60:151–160
Obuekwe CO, Al-Jadi ZK, Al-Saleh ES (2009) Hydrocarbon degradation in relation to cell-surface hydrophobicity among bacterial hydrocarbon degraders from petroleum-contaminated Kuwait desert environment. Int Biodeterior Biodegradation 63:273–279
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Stenstrom TA (1989) Bacterial hydrophobicity an overall parameter for the measurement of adhesion potential to soil particles. Appl Environ Microbiol 55:142–147
Van Loosdrecht MCM, Lyklema J, Norde W, Scharaa G, Zehnder AJB (1987) The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol 53:1893–1897
Zeng GM, Zhong H, Huang GH, Fu HY (2005) Physicochemical and microbiological effects of biosurfactant on the remediation of HOC-contaminated soil. Prog Nat Sci 15:578–585
Zhang YM, Miller RM (1994) Effect of a Pseudomonas rhamnolipid biosurfactant on cell hydrophobicity and biodegradation of octadecane. Appl Environ Microbiol 60:2101–2106
Zhong H, Zeng GM, Liu JX, Xu XM, Yuan XZ, Fu HY, Huang GH, Liu ZF, Ding Y (2008) Adsorption of monorhamnolipid and dirhamnolipid on two Pseudomonas aeruginosa strains and the effect on cell surface hydrophobicity. Appl Microbiol Biotechnol 79:671–677
Acknowledgments
This study was supported by research grant No. N N304 163337, Polish Ministry of Science and Higher Education.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kaczorek, E., Jesionowski, T., Giec, A. et al. Cell surface properties of Pseudomonas stutzeri in the process of diesel oil biodegradation. Biotechnol Lett 34, 857–862 (2012). https://doi.org/10.1007/s10529-011-0835-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0835-x