Abstract
Biodegradation of water insoluble hydrocarbons can be significantly increased by the addition of natural surfactants one. Very promising option is the use of saponins. The obtained results indicated that in this system, after 21 days, 92% biodegradation of diesel oil could be achieved using Pseudomonas alcaligenes. No positive effect on the biodegradation process was observed using synthetic surfactant Triton X-100. The kind of carbon source influences the cell surface properties of microorganisms. Modification of the surface cell could be observed by control of the sedimentation profile. This analytical method is a new approach in microbiological analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Petroleum hydrocarbons are potential sources of soil and water contamination. They penetrate the environment through industrial leaks and spills, leaks from ships or tankers or other transporting devices. There are many various clean-up technologies. These can be categorized in three general schemes: chemical, physical and biological (Kermanshahi et al. 2005). Bioremediation is believed to be one of the main processes used in the cleaning-up of contaminated soil or groundwater. This method is efficient and economical (Obire and Nwaubeta 2001; Yu et al. 2005; Penet et al. 2004). Many organic compounds have limited water solubility and their bioavailability for microorganisms is very low this therefore often limits the rate of biodegradation in soil and aqueous systems. The uptake of hydrocarbon by microorganisms is possible in three different mechanisms: hydrocarbon dissolved in the aqueous phase (Bouchez et al. 1995; Mulder et al. 1998), direct cell contact and the uptake of hydrocarbon drops (Stelmack et al. 1999; Kim et al. 2002; Bouchez-Naitali et al. 2001) and the uptake of pseudosolubilized hydrocarbon droplets by using biosurfactant (Goswami and Singh 1991; Shreve et al. 1995). Which mechanisms is used to uptake of organic compounds is depended of microorganism that takes part in the bioremediation process. The utilization of hydrocarbon will be aided by improved contact between hydrophobic organic compounds and the cells. The ability to adhere on to hydrocarbon is correlated with cell surface hydrophobicity (CSH). Statistical analyses showed a significantly high correlation between the ability of crude oil degradation and CSH (Obuekwe et al. 2009). An addition of surfactants is a promising approach to increase hydrocarbon biodegradation (Liu et al. 1995; Volkering et al. 1998; Noordman and Janssen 2002). Surfactants can increase solubilization and dispersion of hydrocarbon as well as be able to change the affinity between a microbial cell and an organic compound. The introduction of some synthetic surfactants into the environment may be dangerous due to their toxicity and potential degradation products. For this reason is the use of safe surfactants for environmental bioremediation of great importance. Selection of surfactants for bioremediation application could be based on parameter estimation and experimental data, taking in consideration physico-chemical characteristics, toxicity and effectiveness of biodegradation (Franzetti et al. 2008). Introduction to the environmental system of biologically produced surfactants is a better solution. Biosurfactants are often produced by bacterial strains growing on hydrocarbons. They stimulate the growth of these bacteria and accelerate bioremediation. Microorganisms which produce biosurfactants are common in nature and can degrade different types of hydrocarbons: short-chained, long-chained and numerous aromatic compounds, including polycyclic aromatic hydrocarbons (Ron and Rosenberg 2002). Biosurfactants, due to their biodegradability have an environmentally friendly nature. These compounds are nontoxic, have low adsorption onto soil and good solubilization efficiency (Paria 2008). They could enhance the affinity of microorganism cells to hydrophobic organic compounds. The bacterial cell surface has a fundamental role in their adhesion to surfaces (Bruinsma et al. 2001). Bacterial cells can play a role in stabilizing emulsions in circumstances ranging from oil spills to fermentation (Dorobantu et al. 2004). Surfactants can adsorb on to bacterial cells and change the cell surface hydrophobicity or increase the membrane permabilization. The measurement of microbial adhesion to the surface can be determined by different methods: microbial adhesion to hydrocarbon (MATH), hydrophobic interaction chromatography (HIC) or contact angle measurements. However, a correlation between these methods can be observed only for some microbial strains (van der Mei et al. 1995).
The aim of our research was to determine the influence of different amounts of surfactants (rhamnolipides, saponins and Triton X-100) on the cell surface properties of the Pseudomonas alcaligenes S22 strain. Cell surface adhesion to hydrocarbon was assessed by using the MATH assay and the sedimentation profile. The influence of surfactants on diesel oil biodegradation was also determined.
Materials and methods
Microorganisms and growth conditions for the biodegradation test
The microbial strain Pseudomonas alcaligenes S22—(biochemical profile: 00472067073) was used in the experiments. The identification of the bacterial strain was performed using biochemical tests ID 32 GN (prod. bio-Merieoux, France) and molecular techniques. The strains were isolated from soil contaminated with crude oil. The culture medium used throughout these studies consisted of (g l−1): Na2HPO4 × 2H2O 7.0, KH2PO4 2.8, NaCl 0.5, NH4Cl 1.0, MgSO4 × 7H2O 0.01, FeSO4 × 7H2O 0.001, MnSO4 × 4H2O 0.0005, ZnCl2 0.00064, CaCl2 × 6H2O 0.0001, BaCl2 0.00006, CoSO4 × 7H2O 0.000036, CuSO4 × 5H2O 0.000036, H3BO3 0.00065, EDTA 0.001, and HCl 37% 0.0146 ml l−1. The pH of the medium was 7.2. For bacteria stock cultures, yeast extract (0.2 g l−1) was added.
A liquid culture was started by adding a loop full of cells from an agar plate to a 250 ml Erlenmeyer flask containing 50 ml of medium. After approximately 24 h a few ml (in the range 3–5 ml) of this liquid culture was used for the inoculation of the final culture to reach an OD of ca. 0.1 (this corresponds 1 × 108 cells per ml). The microbial growth (bacteria, yeast) was monitored through culture densities, measuring absorption spectrophotometrically at 620 nm (data not given).
Surfactants
The following surface active agents were used:
-
Rhamnolipides, glycolipids produced by Pseudomonas aeruginosa, anionic surface active compounds, available on the market as JBR 425, Jeneil Biosurfactant Company, USA.
-
Saponins, glycosides, non ionic natural surfactant, extracted from Yucca and Quillaya desert plants, Quillaya Bark (Sigma–Aldrich).
-
Triton X-100—polyethylene glycol tert-octylphenyl ether (Sigma–Aldrich).
Biodegradation test
Diesel oil was used for estimating the influence of surfactants on biodegradation. Hydrocarbon concentration in the experiments was 2% (w/v). Surfactants were used at 120 mg l−1 concentrations. This quantity of surfactants was chosen earlier experimentally. Each experiment was repeated five times and the biodegradation values were calculated as a mean value out of five flasks with a precision of ±4.6%. Hydrocarbon content was determined using the “standard method for gravimetric determination of hydrocarbon” (PN-86 C-04573/01). Biodegradation was calculated as (X 0 – X 1)/X 0 100% (%), where X 0 is the initial amount of hydrocarbon, X 1 the amount of hydrocarbon after biodegradation. Samples were incubated at 25°C and shaken at 120 rpm for 7, 14 and 21 days. After the biodegradation process, the whole cultivation broth was centrifuged to separate biomass. The residual aqueous phase was subjected to the double extractions with ethyl ether. The final results were calculated with the respect to blank samples (hydrocarbon with medium, without microorganisms).
Microbial adhesion to hydrocarbons
Microbial surface hydrophobicity was assessed with a microbial adhesion to the hydrocarbon method (MATH). The greater adhesion to hydrocarbon the greater hydrophobicity. Experiments were carried out using the procedure described by Rosenberg et al. (1980). The culture was grown on different carbon sources: a model mixture of dodecane and hexadecane, diesel oil, glucose, different surfactant concentrations and in diesel oil-surfactant system. Cells in the exponential phase were centrifuged at 8,000 g for 5 min and washed twice with a PUM buffer to remove residual hydrocarbons. The PUM buffer contained: 19.7 g l−1 K2HPO4, 7.26 g l−1 KH2PO4, 1.8 g l−1 H2NCONH2 and 0.2 g l−1 MgSO4 7H2O. Washed cells were resuspended in the PUM buffer to fit an optical density of ca. 1.0 (A 0). Optical density was measured at 600 nm on UV–Visible Spectrophometer Shimadzu. Next, 500 μl of hydrocarbon was added to 5 ml of microbial suspension and vortexed for 2 min. After 10 min the optical density of the aqueous phase was measured (A 1). Bacterial adhesion to hydrocarbon is calculated as [1 – (A 0 – A 1)/A 0] × 100% (%). Each experiment was repeated three times, and values for cell adhesion to hydrocarbon were calculated as a mean value out of three flasks with a precision of ±1.2%.
Sedimentation profile
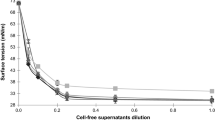
The sedimentation profile was measured on the K-100 tensiometer (KRŰSS), which contained an equipment and computer program for recording the sedimentation profile of particles. The K-100 instrument was used for a suspended probe to directly measure the mass sediment time. After 7 days, cells in the exponential phase were centrifuged at 8,000 g for 5 min and washed twice with a PUM buffer. Next, samples were prepared, to be measured in the following way: 0.1 g of bacterial cells was suspended in 75 ml of PUM buffer. This solution was mixed for 120 s directly before measurement with a magnetic stirrer and then the mass of sedimentation cells were immediately measured. Sedimentation of the parent strain (before modification) is a blank sample. The sedimentation is correlated with a gradient of the sedimentation profile in the first few seconds. Faster particle sedimentation is registered as a higher gradient of the sedimentation profile.
Results and discussion
The Pseudomonas alkaligenes S22 strain was isolated from crude oil contaminated soil by an enrichment culture technique. The initial bacterial adhesion to hydrocarbon was only 8.4% and was comparable with the cell surface adhesion in a glucose system. An increase of cell surface adhesion to hydrocarbon was observed when hydrophobic carbon sources were added to the system. In the case when the model mixture of hydrocarbons (dodecane and hexadecane 1:1 w/w) was present in the system the adhesion to hydrocarbon was 19.2%; however a higher adhesion to hydrocarbon (31.4%) was recorded when diesel oil was in the system (Table 1).
Only 0.02% of crude oil is soluble in water, therefore, the emulsification of oil is necessary for improving the bioavaibility of insoluble hydrocarbon to the microorganism (Gerson 1993). An addition of surfactants to the system changed the cell surface properties of the tested strain. The modification of the cell surface depends on the kind and amount of surfactant (Fig. 1). Only for Triton X-100 was the higher adhesion to hydrocarbon observed than for the parent strain. For saponins up to 120 mg/l, a decrease of adhesion to hydrocarbon was registered and then, an increase to the value of 24%. Rhamnolipides did not cause any significant changes in the system (Fig. 1a). There is a difference between bacterial cell adhesion to hydrocarbon measured in systems where hydrocarbons are supplemented with surfactants and those systems with surfactants alone. This is especially observed in the case of natural surfactants: rhamnolipides and saponins (Fig 1b). Synthetic Triton X-100 caused a decrease in adhesion to hydrocarbon in the system with hydrocarbon. Surfactants increase the interfacial area between the aqueous-insoluble hydrocarbons. Beal and Betts (2000) suggested that surfactants only act to enhance the uptake of hydrophobic hydrocarbons in Ps. aeruginosa and are not required for the actual process to take place.
The sedimentation profile of bacterial strains depends on the kind of carbon source present in the system (Fig. 2). When cell surface of bacteria became more hydrophobic probably could be supposed, that a tendency for agglomeration of cells is observed and a rapid sedimentation is recorded. Bigger particles rapidly settle in the system and the values of the sedimentation mass are bigger than in the case of more hydrophilic bacteria cells. A higher gradient of sedimentation profile, almost for all tested systems, results in bigger values of the sedimentation mass.
The obtained results showed that higher sedimentation masses were observed in the diesel oil system than in the hydrocarbon system. In these two systems Pseudomonas alcaligenes S22 had different surface cell properties, which confirm the result obtained by the MATH method. In the diesel oil system the adhesion to hydrocarbon of this strain was the highest. Moreover, a higher gradient of the sedimentation profile in first two-seconds was observed in the diesel oil system than in the hydrocarbon system. The results of the sedimentation test for Pseudomonas alcaligenes S22, grown on glucose, showed smaller sedimentation masses, which is probably connected with the more hydrophilic properties of surface cell of bacteria. In the case of a model mixture of hydrocarbon the sedimentation mass was insignificantly less than for the parent bacterial strain. However, the gradient of the sedimentation profile was almost the same in an initial period of sedimentation. The addition of surfactants (120 mg/l) to the system with bacteria caused the modification of cellular membranes and the size of particles which reflects on different sedimentation profiles (Fig. 2b). This is especially visible for the system with hydrocarbons. In the case of the addition only surfactants (in the system without hydrocarbon) the comparable masses of cell sedimentation were observed. In general the addition of surfactants caused a decrease in the sedimentation mass of the bacteria cell. The sedimentation profile can be a good analytical method, which allows the observation of the modifications of the surface cells of microorganisms, though only in systems of bacteria:surfactant and bacteria:hydrocarbons. The use of surfactants result in forming aggregates in the system with bacteria:hydrocarbons:surfactant which disrupts the determination of microorganism hydrophobicity using sedimentation method. This method has not yet been used in this type of investigation and apart from microbial adhesion to hydrocarbon could be an additional method for the observation of changes in the cell surface. According to Rosenberg et al. (1980) the MATH method is useful in enabling the separation of certain cell mixtures, and in separating hydrophobic cell components. It can be used for the determination of the relationship between the metabolism of hydrophobic substrates and the modification of cell surface hydrophobicity. However, van der Mei et al. (1995) noted, that MATH remains useful as a simple assay for studying the adhesion of bacteria to the hydrophobic surface which is essentially different from being a hydrophobicity assay. In our opinion an increase of cell surface adhesion to hydrocarbon is connected with an increase in the hydrophobicity of the tested strain.
Results of diesel oil biodegradation showed that Pseudomonas alcaligenes S22 is a good biological agent. The experiment was carried out for 21 days. Biodegradation was measured after every 7 days. After 21 days, 70% diesel oil biodegradation was noticed. The largest differences were observed between 7 and 14 days, when biodegradation increased about 100% (Fig. 3). The addition of natural surfactants to the diesel oil system caused an increase in the biodegradation. Saponins turned out to be the most effective. The biodegradation of diesel oil was 52% after 7 days. In the following days biodegradation increased and finally 92% of degradation was observed. The adhesion to hydrocarbon in these systems increased insignificantly during the process (Fig. 4). In the case of rhamnolipides the biodegradation of diesel oil was 80% after 21 days. For rhamnolipides, as well as for Triton X-100, changes in adhesion to hydrocarbons after 14 and 21 days were observed. After 21 days of the biodegradation process, the cell surface of tested strain had lower adhesion properties than after 7 days. According to Zhang and Miller (1994), a mutual attraction between biosurfactant and microbial cells can lead to an increase in cell hydrophobicity, and therefore cells have a better contact with the hydrophobic substrate finally achieving a higher biodegradation.
After 7 days of process, our obtained results confirmed this observation. The longer time of experiments had effects on the adhesion properties of the tested strains. It is especially noticeable in Triton X-100–diesel oil and rhamnolipides–diesel oil systems. In these cases different interactions between the bacterial cell and both surfactants (rhamnolipides and Triton X-100) were observed than for saponins. For rhamnolipides the decrease in bacterial adhesion to hydrocarbons after 7 days led to a higher biodegradation. However, for Triton X-100 a difference was noticed; the decrease of bacterial adhesion to hydrocarbon stopped the progress of biodegradation.
Our previous research (Chrzanowski et al. 2006) suggested, that rhamnolipids adsorb on the microbial cell surface and change their surface properties. Saponins, moreover build in the membrane lipophilic part and increase membrane permeability (Leung 1997).
Mohanty and Mukherji (2007) suggested that Triton X-100 can significantly enhance the rate and extent of diesel degradation by Exiguobacterium aurantiacum and Burkholderia cepacia. They obtained these results when the Triton X-100 concentration was twice CMC. Cserhati et al. (2002) suggested that Triton X-100 rather changes the cell growth of microorganisms in the presence of a high concentration organic compounds. This surfactant decreases the degree of aggregation and improves growth. However, the use of natural surfactants is better, favorable to the environment, and are effective in low concentrations. The addition of surfactants to disperse oil have a negative effect on hydrocarbon biodegradation by a microorganism, which uptake hydrocarbon by direct contact with the oil phase (Hori et al. 2002). For this type of bacterium, surfactant must be used at a level over CMC. Therefore, before application any surfactants, hydrocarbon uptake mechanisms of the dominate degrader should be defined.
Conclusion
Isolated from the environment Pseudomonas alcaligenes S22 strain has hydrophilic properties. During growing on hydrophobic carbon source the increase in adhesion to hydrocarbon was observed. The addition of surfactants to the system changed crucial parameters and the cell surface properties of microorganisms. Surfactants also caused an increase in hydrocarbon biodegradation. A very promising approach is the use of saponins. In this case a 92% degree of diesel oil biodegradation was obtained. The use of sedimentation profiles of bacterial strains seem to be a new and good analytical method which enables recording the modification of the surface cell of microorganisms, though only for the systems: bacteria:surfactant and bacteria:hydrocarbons.
References
Beal R, Betts WB (2000) Role of rhamnolipid biosurfactants in the uptake and mineralization of hexadecane in Pseudomonas aeruginosa. J Appl Microbiol 89:158–168
Bouchez M, Blanchet D, Vandecasteele JP (1995) Substrate availability in phenanthrene biodegradation: transfer mechanisms and influence on metabolism. Appl Microbiol Biotechnol 43:952–960
Bouchez-Naitali M, Blanchet D, Dardin D, Vandecasteele JP (2001) Evidence for interfacial uptake in hexadecane degradation by Rhodococcus equi: the importance of cell flocculation. Microbiol SGM 147:2537–2543
Bruinsma GM, Rustema-Abbing M, van der Mei HC, Busscher HJ (2001) Effects of cell surface damage on surface properties and adhesion of Pseudomonas aeruginosa. J Microbiol Methods 45:95–101
Chrzanowski Ł, Kaczorek E, Pijanowska A, Olszanowski A (2006) The relation between rhamnolipid adsorption on yeast and bacterial strains, hydrophobicity and hydrocarbon biodegradation. Fresen Environ Bull 15:682–686
Cserhati T, Forgacs E, Oros G (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28:337–348
Dorobantu LS, Yeung AKC, Foght JM, Gray MR (2004) Stabilization of oil–water emulsions by hydrophobic bacteria. Appl Environ Micro 70:6333–6336
Franzetti A, Di Gennaro P, Bestetti G, Lasagni M, Pitea D, Collina E (2008) Selection of surfactants for enhancing diesel hydrocarbons-contaminated media bioremediation. J Hazard Material 152:1309–1316
Gerson DF (1993) The biophysics of microbial 1 surfactants: growth on insoluble substrates. In: Kozaric N (ed) Surfactant science series, biosurfactants: production, properties, applications. Marcel Dekker, New York, USA, pp 269–286
Goswami P, Singh HD (1991) Different modes of hydrocarbon uptake by two Pseudomonas species. Biotechnol Bioeng 37:1–11
Hori K, Matsuzaki Y, Tanji Y, Unno H (2002) Effect of dispersing oil phase on the biodegradability of a solid alkane dissolved in non-biodegradable oil. Appl Microbiol Biotechnol 59:574–579
Kermanshahi Pour A, Karamanev D, Margaritis A (2005) Biodegradation of petroleum hydrocarbons in an immobilized cell airlift bioreactor. Water Res 39:3704–3714
Kim IS, Foght JM, Gray MR (2002) Selective transport and accumulation of alkanes by Rhodococcus erythropolis S + 14He. Biotechnol Bioeng 80:650–658
Leung YM (1997) Specific interaction between tetrandrine and Quillaja saponins in promoting permeabilization of plasma membrane in human leukemic HL-60 cell. Biomembrane 1325:318–328
Liu Z, Jacobson AM, Luthy RG (1995) Biodegradation of naphthalene in aqueous non-ionic surfactant system. Appl Environ Microbiol 61:145–151
Mohanty G, Mukherji S (2007) Effect of an emulsifying surfactant on diesel degradation by cultures exhibiting inducible cell surface hydrophobicity. J Chem Technol Biotechnol 82:1004–1011
Mulder H, Breure AM, Andel JGV, Grotenhuis JTC, Rulkens WH (1998) Influence of thermodynamic conditions on naphthalene dissolution and subsequent biodegradation. Biotechnol Bioeng 57:145–154
Noordman WH, Janssen DB (2002) Rhamnolipids stimulates uptake of hydrophobic compounds by Pseudomonas aeruginos. Appl Environ Microbiol 68:4502–4508
Obire O, Nwaubeta O (2001) Biodegradation of refined petroleum hydrocarbons in soil. J Appl Sci Technol 5:43–46
Obuekwe ChO, Al-Jadi ZK, Al-Saleh ES (2009) Hydrocarbon 1 degradation in relation to cell surface hydrophobicity among bacterial hydrocarbon degraders from petroleum contaminated Kuwait desert environment. Int Biodeter Biodegrad 63:273–279
Paria S (2008) Surfactant-enhanced remediation of organic contaminated soil and water. Adv Colloid Inteface Sci 138:24–58
Penet S, Marchal R, Sghir A, Monot F (2004) Biodegradation of hydrocarbon cuts used for diesel oil formulation. Appl Microbiol Biotechnol 66:40–47
Ron EZ, Rosenberg E (2002) Biosurfactants and oil bioremediation. Curr Opin Biotechnol 13:249–252
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Shreve GS, Inguva S, Gunnam S (1995) Rhamnolipid biosurfactant enhancement of hexadecane biodegradation by Pseudomonas aeruginosa. Mol Mar Biol Biotechnol 4:331–337
Stelmack PL, Gray MR, Pickard MA (1999) Bacterial adhesion to soil contaminants in the presence of surfactant. Appl Environ Microbiol 65:163–168
Van der Mei HC, van de Belt-Gritter B, Busscher HJ (1995) Implication of microbial adhesion to hydrocarbons for evaluating cell surface hydrophobicity 2. Adhesion mechanisms. Colloid Surface B 5:117–126
Volkering F, Breure AM, Rulkens WH (1998) Microbiological aspects of surfactants use for biological soil remediation. Biodegradation 8:401–417
PN-86 C-04573/01—Polish standard method for gravimetric determination of hydrocarbon. Polish Committee for Standaraization, Warszawa
Yu KSH, Wong AHY, Yau KWY, Wong YS, Tam NFY (2005) Natural attenuation, biostimulation and bioaugmentation on biodegradation of polycyclic aromatic hydrocarbons (PAHs) in mangrove sediments. Mar Pol Bulletin 51:1071–1077
Zhang Y, Miller RM (1994) Effect of a Pseudomonas rhamnolipides biosurfactant on cell hydrophobicity and biodegradation of octadecane. Appl Environ Microbiol 60:2101–2106
Acknowledgments
This study was funded by the Polish Ministry of Science and Higher Education (GR 32/0897/2008).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kaczorek, E., Moszyńska, S. & Olszanowski, A. Modification of cell surface properties of Pseudomonas alcaligenes S22 during hydrocarbon biodegradation. Biodegradation 22, 359–366 (2011). https://doi.org/10.1007/s10532-010-9406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-010-9406-4