Abstract
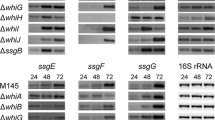
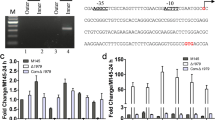
Bacterial integration host factors (IHFs) play important roles in site-specific recombination, DNA replication, transcription, genome organization and bacterial pathogenesis. In Streptomyces coelicolor, there are three putative IHFs: SCO1480, SCO2950 and SCO5556. SCO1480 or Streptomyces IHF (sIHF) was previously identified as a transcription factor that binds to the promoter region of redD, the pathway-specific regulatory gene for the undecylprodigiosin biosynthetic gene cluster. Here we show that production of the pigmented antibiotics actinorhodin and undecylprodigiosin is strongly enhanced in sihf null mutants, while sporulation was strongly inhibited, with an on average 25% increase in spore size. Furthermore, the sihf mutant spores showed strongly reduced viability, with high sensitivity to heat and live/dead staining revealing a high proportion of empty spores, while enhanced expression of sIHF increased viability. This suggests a major role for sIHF in controlling viability, perhaps via the control of DNA replication and/or segregation. Proteomic analysis of the sihf null mutant identified several differentially expressed transcriptional regulators, indicating that sIHF may have an extensive response regulon. These data surprisingly reveal that a basic architectural element conserved in many actinobacteria such as mycobacteria, corynebacteria, streptomycetes and rhodococci may act as a global regulator of secondary metabolism and cell development.






Similar content being viewed by others
References
Aviv M, Giladi H, Schreiber G, Oppenheim AB, Glaser G (1994) Expression of the genes coding for the Escherichia coli integration host factor are controlled by growth phase, rpoS, ppGpp and by autoregulation. Mol Microbiol 14(5):1021–1031
Bagchi S, Tomenius H, Belova LM, Ausmees N (2008) Intermediate filament-like proteins in bacteria and a cytoskeletal function in Streptomyces. Mol Microbiol 70(4):1037–1050
Barends S, Zehl M, Bialek S, de Waal E, Traag BA, Willemse J, Jensen ON, Vijgenboom E, van Wezel GP (2010) Transfer-messenger RNA controls the translation of cell-cycle and stress proteins in Streptomyces. EMBO Rep 11(2):119–125
Benson DA, Boguski MS, Lipman DJ, Ostell J, Ouellette BF (1998) GenBank. Nucleic Acids Res 26(1):1–7
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417(6885):141–147
Bibb M (1996) 1995 Colworth Prize Lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142(Pt 6):1335–1344
Bibb MJ (2005) Regulation of secondary metabolism in Streptomycetes. Curr Opin Microbiol 8(2):208–215
Bierman M, Logan R, O’Brien K, Seno ET, Rao RN, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116(1):43–49
Boubrik F, Bonnefoy E, Rouviere-Yaniv J (1991) HU and IHF: similarities and differences. In Escherichia coli, the lack of HU is not compensated for by IHF. Res Microbiol 142(2–3):239–247
Chakraburtty R, Bibb M (1997) The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol 179(18):5854–5861
Chang HM, Chen MY, Shieh YT, Bibb MJ, Chen CW (1996) The cutRS signal transduction system of Streptomyces lividans represses the biosynthesis of the polyketide antibiotic actinorhodin. Mol Microbiol 21(5):1075–1085
Charlier D, Hassanzadeh G, Kholti A, Gigot D, Pierard A, Glansdorff N (1995) carP, involved in pyrimidine regulation of the Escherichia coli carbamoylphosphate synthetase operon encodes a sequence-specific DNA-binding protein identical to XerB and PepA, also required for resolution of ColEI multimers. J Mol Biol 250(4):392–406
Chater KF (2001) Regulation of sporulation in Streptomyces coelicolor A3(2): a checkpoint multiplex? Curr Opin Microbiol 4(6):667–673
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393(6685):537–544
den Hengst CD, Tran NT, Bibb MJ, Chandra G, Leskiw BK, Buttner MJ (2010) Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol Microbiol 78(2):361–379
Devroede N, Huysveld N, Charlier D (2006) Mutational analysis of intervening sequences connecting the binding sites for integration host factor, PepA, PurR, and RNA polymerase in the control region of the Escherichia coli carAB operon, encoding carbamoylphosphate synthase. J Bacteriol 188(9):3236–3245
Ditto MD, Roberts D, Weisberg RA (1994) Growth phase variation of integration host factor level in Escherichia coli. J Bacteriol 176(12):3738–3748
Flardh K, Buttner MJ (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7(1):36–49
Goosen N, van de Putte P (1995) The regulation of transcription initiation by integration host factor. Mol Microbiol 16(1):1–7
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA 100(4):1541–1546
Guthrie EP, Flaxman CS, White J, Hodgson DA, Bibb MJ, Chater KF (1998) A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiology 144(Pt 3):727–738
Hengge-Aronis R (1996) Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol Microbiol 21(5):887–893
Kano Y, Ogawa T, Ogura T, Hiraga S, Okazaki T, Imamoto F (1991) Participation of the histone-like protein HU and of IHF in minichromosomal maintenance in Escherichia coli. Gene 103(1):25–30
Karp PD, Riley M, Saier M, Paulsen IT, Collado-Vides J, Paley SM, Pellegrini-Toole A, Bonavides C, Gama-Castro S (2002) The EcoCyc database. Nucleic Acids Res 30(1):56–58
Kieser T, Bibb MJ, Buttner MJ, Chater K, Hopwood DA (2000) Practical Streptomyces genetics. Mol Microbiol, vol 3. John Innes Centre, Norwich Research Park, Colney, Norwich
Lawlor EJ, Baylis HA, Chater KF (1987) Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2). Genes Dev 1(10):1305–1310
Lee CJ, Won HS, Kim JM, Lee BJ, Kang SO (2007) Molecular domain organization of BldD, an essential transcriptional regulator for developmental process of Streptomyces coelicolor A3(2). Proteins 68(1):344–352
Luirink J, von Heijne G, Houben E, de Gier JW (2005) Biogenesis of inner membrane proteins in Escherichia coli. Annu Rev Microbiol 59:329–355
Mangan MW, Lucchini S, Danino V, Croinin TO, Hinton JC, Dorman CJ (2006) The integration host factor (IHF) integrates stationary-phase and virulence gene expression in Salmonella enterica serovar Typhimurium. Mol Microbiol 59(6):1831–1847
Nicholas HB Jr, Ropelewski AJ, Deerfield DW II (2002) Strategies for multiple sequence alignment. BioTechniques 32(3):572–574, 576, 578 passim
Ohnishi Y, Yamazaki H, Kato JY, Tomono A, Horinouchi S (2005) AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci Biotechnol Biochem 69(3):431–439
Park SS, Yang YH, Song E, Kim EJ, Kim WS, Sohng JK, Lee HC, Liou KK, Kim BG (2009) Mass spectrometric screening of transcriptional regulators involved in antibiotic biosynthesis in Streptomyces coelicolor A3(2). J Ind Microbiol Biotechnol 36(8):1073–1083
Pedulla ML, Hatfull GF (1998) Characterization of the mIHF gene of Mycobacterium smegmatis. J Bacteriol 180(20):5473–5477
Pedulla ML, Lee MH, Lever DC, Hatfull GF (1996) A novel host factor for integration of mycobacteriophage L5. Proc Natl Acad Sci USA 93(26):15411–15416
Piette A, Derouaux A, Gerkens P, Noens EE, Mazzucchelli G, Vion S, Koerten HK, Titgemeyer F, De Pauw E, Leprince P, van Wezel GP, Galleni M, Rigali S (2005) From dormant to germinating spores of Streptomyces coelicolor A3(2): new perspectives from the crp null mutant. J Proteome Res 4(5):1699–1708
Rice PA (1997) Making DNA do a U-turn: IHF and related proteins. Curr Opin Struct Biol 7(1):86–93
Rigali S, Nothaft H, Noens EE, Schlicht M, Colson S, Muller M, Joris B, Koerten HK, Hopwood DA, Titgemeyer F, van Wezel GP (2006) The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol 61(5):1237–1251
Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, van Wezel GP (2008) Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep 9(7):670–675
Salerno P, Larsson J, Bucca G, Laing E, Smith CP, Flardh K (2009) One of the two genes encoding nucleoid-associated HU proteins in Streptomyces coelicolor is developmentally regulated and specifically involved in spore maturation. J Bacteriol 191(21):6489–6500
Strauch E, Takano E, Baylis HA, Bibb MJ (1991) The stringent response in Streptomyces coelicolor A3(2). Mol Microbiol 5(2):289–298
Streptomyces database website. http://strepdb.streptomyces.org.uk/
Takeuchi A, Matsumura H, Kano Y (2002) Cloning and expression in Escherichia coli of a gene, hup, encoding the histone-like protein HU of Bifidobacterium longum. Biosci Biotechnol Biochem 66(3):598–603
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Thuy ML, Kharel MK, Lamichhane R, Lee HC, Suh JW, Liou K, Sohng JK (2005) Expression of 2-deoxy-scyllo-inosose synthase (kanA) from kanamycin gene cluster in Streptomyces lividans. Biotechnol Lett 27(7):465–470
Uguru GC, Stephens KE, Stead JA, Towle JE, Baumberg S, McDowall KJ (2005) Transcriptional activation of the pathway-specific regulator of the actinorhodin biosynthetic genes in Streptomyces coelicolor. Mol Microbiol 58(1):131–150
van Wezel GP, McDowall KJ (2011) The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat Prod Rep 28(7):1311–1333
van Wezel GP, McKenzie NL, Nodwell JR (2009) Chapter 5. Applying the genetics of secondary metabolism in model actinomycetes to the discovery of new antibiotics. Methods Enzymol 458:117–141
Willemse J, van Wezel GP (2009) Imaging of Streptomyces coelicolor A3(2) with reduced autofluorescence reveals a novel stage of FtsZ localization. PLoS One 4(1):e4242
Willemse J, Borst JW, de Waal E, Bisseling T, van Wezel GP (2011) Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces. Genes Dev 25(1):89–99
Yang YH, Joo HS, Lee K, Liou KK, Lee HC, Sohng JK, Kim BG (2005) Novel method for detection of butanolides in Streptomyces coelicolor culture broth, using a His-tagged receptor (ScbR) and mass spectrometry. Appl Environ Microbiol 71(9):5050–5055
Yang YH, Song E, Kim EJ, Lee K, Kim WS, Park SS, Hahn JS, Kim BG (2009) NdgR, an IclR-like regulator involved in amino-acid-dependent growth, quorum sensing, and antibiotic production in Streptomyces coelicolor. Appl Microbiol Biotechnol 82(3):501–511
Yang YH, Song E, Lee BR, Kim EJ, Park SH, Kim YG, Lee CS, Kim BG (2010) Rapid functional screening of Streptomyces coelicolor regulators by use of a pH indicator and application to the MarR-like regulator AbsC. Appl Environ Microbiol 76(11):3645–3656
Acknowledgments
This work was supported by WCU (World Class University) program (R322009000102130), NRL (National Research Lab) program (20090083035), and Basic Science Research Program (2010-0009942) through the National Research Foundation (NRF) grant funded by the Korean government (MEST), and by a VICI grant from the Dutch applied research council (STW) to GPvW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yung-Hun Yang and Eunjung Song have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, YH., Song, E., Willemse, J. et al. A novel function of Streptomyces integration host factor (sIHF) in the control of antibiotic production and sporulation in Streptomyces coelicolor . Antonie van Leeuwenhoek 101, 479–492 (2012). https://doi.org/10.1007/s10482-011-9657-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9657-z