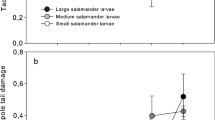
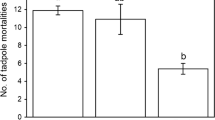
Abstract
Global warming may induce significant changes in species life history traits particularly in amphibians, which are characterized by complex and plastic life cycles. Because both warming and predators are often suggested to reduce size at metamorphosis in amphibians, we hypothesized that the size at metamorphosis was further reduced by experimental warming in the presence of predators. We conducted a factorial-designed experiment involving two factors and two levels (warmed vs. ambient, lethal predator absence vs. presence, resulting in four treatments) using Rana kukunoris tadpoles in the eastern Tibetan Plateau, and we examined its behavioral, growth, and developmental responses to warming in the presence and absence of predatory beetles (Agabus sp.) for 13 weeks. During the course of the experiment, a similar level of tadpole mortality due to the diving beetles was found between ambient and warmed treatments, but the warming effect on size at metamorphosis depended on whether the predators were present or absent. In the absence of predators, warming did not significantly increase tadpole growth but advanced the timing of metamorphosis, such that size at metamorphosis of forelimb emergence and tail resorption was much reduced in terms of body fresh weight. In the presence of predators, warming increased tadpole growth rate much more than the development rate (as reflected by duration of the tadpole stage), and therefore the size at metamorphosis was significantly increased. The significant effect of the interaction between predator and warming on the size at metamorphosis could be attributed to the tadpole response in the frequencies of feeding, resting, and swimming to the predator activity level, which was in turn increased by warming. We suggest that warming-induced changes in life history traits should be studied in relation to species interaction so as to accurately predict ecological response of amphibians to the future warmed world.





Similar content being viewed by others
References
Alford RA, Harris RN (1988) Effects of larval growth history on anuran metamorphosis. Am Nat 131:91–106
Altwegg R, Reyer HU (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Alvarez D, Nicieza AG (2002) Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol 16:640–648
Anholt BR, Werner EE (1995) Interaction between food availability and predation mortality mediated by adaptive behavior. Ecology 76:2230–2234
Atkinson D (1994) Temperature and organism size - a biological law for ectotherms? Adv Ecol Res 25:1–58
Atlas M (1935) The effect of temperature on the development of Rana pipiens. Physiol Zool 8:290–310
Bailey PCE (1988) The effect of density and temperature on the swimming and aggregating behavior of the backswimmer, Anisops deanei (Heteroptera: Notonectidae) and subsequent encounter rate with a sit-and-wait predator. Ethology 77:44–57
Ball SL, Baker RL (1996) Predator-induced life history changes: antipredator behavior costs or facultative life history shifts? Ecology 77:1116–1124
Beebee TJC (1995) Tadpole growth: is there an interference effect in nature? Herpetol J 5:204–205
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35:651–673
Benard MF (2006) Survival trade-offs between two predator-induced phenotypes in pacific treefrogs (Pseudacris Regilla). Ecology 87:340–346
Brattstrom BH (1963) A preliminary review of the thermal requirements of amphibians. Ecology 44:238–255
Brockelman WY (1969) An analysis of density effects and predation in Bufo americanus tadpoles. Ecology 50:632–644
Dai J, Dai Q, Zhang M, Zhang JD, Li C, Liu ZJ, Wang YZ (2005) Habitat selection of three amphibians (Rana kukunoris, Nanorana pleskei, Buf ominshanicus) during summer-autumn in Zoige Wetland National Nature Reserve. Dongwuxue Yanjiu 26:263–271 (In Chinese)
Development Core Team R (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Doherty PA, Wassersug RJ, Lee JM (1998) Mechanical properties of the tadpole tail fin. J Exp Biol 201:2691–2699
Giller PS (1984) Predator gut state and prey detectability using electrophoretic analysis of gut contents. Ecol Entomol 9:157–162
Gollmann B, Gollmann G (1996) Geographic variation of larval traits in the Australian frog Geocrinia victoriana. Herpetologica 52:181–187
Gotthard K (2000) Increased risk of predation as a cost of high growth rate: an experimental test in a butterfly. J Anim Ecol 69:896–902
Gresens SE, Cothran ML, Thorp JH (1982) The influence of temperature on the functional response of the dragonfly Celithemis fasciata (Odonata: Libellulidae). Oecologica 53:281–284
Heiman DR, Knight AW (1975) The influence of temperature on the bioenergetics of the carnivorous stonefly nymph, Acroneuria californica Banks (Plecoptera: Perlidae). Ecology 56:105–116
Heyer WR, McDiarmid RW, Weigmann DL (1975) Tadpoles, predation and pond habitats in the tropics. Biotropica 7:100–111
IPCC (2007) Climate change 2007: synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jayakumar E, Mathavan S (1991) Effect of temperature on the growth and bioenergetics of Laccotrephes griseus (Hemiptera: Nepidae). J Therm Biol 16:93–102
Laurila A, Lindgren B, Laugen AT (2008) Antipredator defenses along a latitudinal gradient in Rana temporaria. Ecology 89:1399–1413
Li P, Zhao E, Dong B (2010) Diversity of amphibians and reptiles in Tibet. Science Press, Beijing, pp 34–35 (in Chinese)
Loman J (2002) Temperature, genetic and hydroperiod effects on metamorphosis of brown frogs Rana arvalis and R. temporaria in the field. J Zool 258:115–129
Lu LH, Bian LG, Zhang ZQ (2011) Climate change: impact on the Arctic, Antarctic and Tibetan Plateau. Adv Polar Sci 22:67–73
Mangel M, Stamps J (2001) Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol Ecol Res 3:583–593
McCollum SA, Van Buskirk J (1996) Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50:583–593
McPeek MA, Peckarsky BL (1998) Life histories and the strengths of species interactions: combining mortality, growth, and fecundity effects. Ecology 79:867–879
MerilaÈ J, Laurila A, Laugen AT, Räsänen K, Pahkala M (2000) Plasticity in age and size at metamorphosis in Rana temporaria—comparison of high and low latitude populations. Ecography 23:457–465
Newman RA (1992) Adaptive plasticity in amphibian metamorphosis. Bioscience 42:671–678
Newman RA (1998) Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level. Oecologia 115:9–16
Newman RA, Dunham AE (1994) Size at metamorphosis and water loss in a desert anuran (Scaphiopus couchii). Copeia 1994:372–381
O’Regan SM, Palen WJ, Anderson SC (2014) Climate warming mediates negative impacts of rapid pond drying for three amphibian species. Ecology 95:845–855
Orizaola G, Dahl E, Nicieza AG, Laurila A (2013) Larval life history and anti-predator strategies are affected by breeding phenology in an amphibian. Oecologia 171:1–9
Peckarsky BL, Cowan CA, Penton MA, Anderson C (1993) Sublethal consequences of stream-dwelling predatory stoneflies on mayfly growth and fecundity. Ecology 74:1836–1846
Qi Y, Felix Z, Dai Q, Wang Y, Yang Y, Wang B, Wang YZ (2007) Post breeding movements, home range, and microhabitat use of plateau brown frogs Rana kukunoris in Zoige Alpine Wetland. Acta Zool Sin 53:974–981 (In Chinese)
Reading CJ (1998) The effect of winter temperatures on the timing of breeding activity in the common toad Bufo bufo. Oecologia 117:469–475
Relyea RA (2001) The relationship between predation risk and antipredator responses in larval anurans. Ecology 82:541–554
Relyea RA (2007) Getting out alive: how predators affect the decision to metamorphose. Oecologia 152:389–400
Rowe L, Ludwig D (1991) Size and timing of metamorphosis in complex life cycles: time constraints and variation. Ecology 72:413–427
Semlitsch RD, Scott DE, Pechmann JHK (1988) Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69:184–192
Semlitsch RD, Pickle J, Parris MJ, Sage RD (1999) Jumping performance and short-term repeatability of newly metamorphosed hybrid and parental leopard frogs (Rana sphenocephala and Rana blairi). Can J Zool 77:748–754
Skelly DK (1994) Activity level and the susceptibility of anuran larvae to predation. Anim Behav 47:465–468
Skelly DK, Werner EE (1990) Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71:2313–2322
Smith DC (1983) Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 64:501–510
Smith DC (1987) Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68:344–350
Smith-Gill SJ, Berven KA (1979) Predicting amphibian metamorphosis. Am Nat 113:563–586
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Climate change 2007: the physical science basis contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge and New York
Stocker TF, Qin DH, Plattner G, Tignor MMB, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (2013) Climate change 2013: the physical science basis. Working group I contribution to the IPCC fifth assessment report. Cambridge University Press, Cambridge
Tejedo M, Marangoni F, Pertoldi C, Richter-Boix A, Laurila A, Orizaola G, Nicieza AG, Álvarez D, Gomez-Mestre I (2010) Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Clim Res 43:31–39
Touchon JC, Warkentin KM (2011) Thermally contingent plasticity: temperature alters expression of predator—induced colour and morphology in a Neotropical treefrog tadpole. J Anim Ecol 80:79–88
Tracy CR, Christian KA, O’Connor MP, Tracy CR (1993) Behavioral thermoregulation by Bufo americanus: the importance of the hydric environment. Herpetologica 49:375–382
Travis J (1984) Anuran size at metamorphosis: experimental test of a model based on intraspecific competition. Ecology 65:1155–1160
Van Buskirk J (1988) Interactive effects of dragonfly predation in experimental pond communities. Ecology 69:857–867
Van Buskirk J (2000) The costs of an inducible defense in anuran larvae. Ecology 81:2813–2821
Van Buskirk J, Anderwald P, Lüpold S, Reinhardt L, Schuler H (2003) The lure effect, tadpole tail shape, and the target of dragonfly strikes. J Herpetol 37:420–424
Vonesh JR, Warkentin KM (2006) Opposite shifts in size at metamorphosis in response to larval and metamorph predators. Ecology 87:556–562
Voss SR (1993) Effect of temperature on body size, developmental stage, and timing of hatching in Ambystoma maculatum. J Herpetol 27:329–333
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Werner EE (1986) Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat 128:319–341
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis nonnormal distributions of competitive ability reflect selection for facultative metamorphosis. Science 182:1305–1314
Wilbur HM, Fauth JE (1990) Experimental aquatic food webs: interactions between two predators and two prey. Am Nat 135:176–204
Wright SJ, Cornejo FH (1990) Seasonal drought and leaf fall in a tropical forest. Ecology 71:1165–1175
Xiang S, Guo R, Wu N, Sun S (2009) Current status and future prospects of Zoige Marsh in eastern Qinghai-Tibet Plateau. Ecol Eng 35:553–562
Zhang W, Li Y (2011) Chinese insects illustrated. Chongqing University Press, Chongqing, pp 263–264 (in Chinese)
Zhao JY, Yang YHS, Xi XQ, Sun SC (2014) Artificial warming facilitates growth but not survival of plateau frog (Rana kukunoris) tadpoles in presence of gape-limited predatory beetles. PLoS ONE. doi:10.1371/journal.pone.0098252
Acknowledgments
We thank Ase Xiaohu for assistance and Lee Frelich for English improvement. This study was supported by National Science Foundation of China (31325004), 973 Program (2013CB956302), and the Ministry of Environmental Protection of China (2011ZX08012-005).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10452_2014_9495_MOESM1_ESM.doc
Online Resource 1 Variation in daily mean temperature during the experiment period. The closed and open symbols stand for warmed and ambient treatments, respectively. (DOC 105 kb)
10452_2014_9495_MOESM2_ESM.doc
Online Resource 2 Tadpole survival during the course of the experiment. W-P-, ambient control; W+P-, warming; W-P+, ambient and predator present; W+P+, warming and predator present. Data are means± 1SE. The difference was significant (P<0.05) between W-P- and W+P- treatments on days 42 and 49 in the absence of predators, as indicated by results of two-way ANOVAs followed by post hoc Tukey’s tests. (DOC 72 kb)
10452_2014_9495_MOESM3_ESM.doc
Online Resource 3 Body fresh weight (a), body length (b) and tail length (c) of surviving tadpoles in four treatments during the course of the experiment. W-P-, ambient control; W+P-, warming; W-P+, ambient and predator-presence; W+P+, warming and predator-presence. The asterisks denote statistically significant differences (*, P<0.05, **, P<0.01, ***, P<0.001) between warmed and ambient treatments in the presence of predators, as derived from a two-way ANOVA followed by post hoc Tukey’s tests on observation days. Data are means±1SE. (DOC 184 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Yang, Y., Xi, X. et al. Artificial warming increases size at metamorphosis in plateau frogs (Rana kukunoris) in the presence of predators. Aquat Ecol 48, 423–434 (2014). https://doi.org/10.1007/s10452-014-9495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-014-9495-y