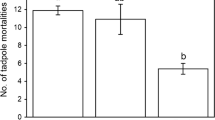
Abstract
Amphibians exhibit extreme plasticity in the timing of metamorphosis, and several species respond to water availability, accelerating metamorphosis when their ponds dry. We analyzed the plasticity of the developmental response to water volume in Rhinella schneideri tadpoles. We raised tadpoles in mesocosm. Covariation between body size at metamorphosis and timing of development was positive. Nevertheless, the first approximately 53% of the metamorphoses finishing the cycle required between 34 and 56 days, and the covariation between body size at metamorphosis and timing of development was negative. For these tadpoles, the larval density and the presence of predators did not significantly affect their mass to metamorphosis. Nevertheless, predators affected time to metamorphosis. For the remainder of the tadpoles that reached metamorphosis at > 56 days, the relationship between body size at metamorphosis and timing of development was positive. For these tadpoles, larval density was important for mass at metamorphosis and presence of predators was also important for time to metamorphosis. Two dominant features were observed: (i) approximately 53% of metamorphs had morphological features similar to individuals developing in desiccating ponds, and (ii) the other individuals had morphological characteristics comparable to metamorphs developing in an unchanging environment.
Similar content being viewed by others
References
Anderson A.R. & Petranka J.W. 2003. Odonate predator does not affect hatching time or morphology of embryos of two amphibians. J. Herpetol. 37(1): 65–71. DOI: 10.1670/0022-1511(2003)037[0065:OPDNAH]2.0.CO;2
Babbit K.J. 2001. Beaviour and growth of southern leopard frog (Rana sphenocephala) tadpoles: effects of food and predation risk. Can. J. Zool. 79(5): 809–814. DOI: 10.1139/z01-040
Benard M.F. 2004. Predator-induced phenotypic plasticity in organism with complex life cycles. Annu. Rev. Ecol. Evol. Syst. 35(2004): 651–673. DOI: 10.1146/annurev.ecolsys.35.021004.112426
Berven K.A. 1990. Factors affecting populations fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71: 1599–1608. DOI: http://dx.doi.org/10.2307/1938295
Bolnick D.I. & Preisser E.L. 2005. Resource competition modifies the strength of trait-mediated predator-prey interactions: a meta-analysis. Ecology 86: 2771–2779. DOI: http://dx.doi.org/10.1890/04-1249
Fusco G. & Minelli A. 2010. Phenotipic plasticity in developmental and evolution: facts and concepts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365(1540): 547–556. DOI: 10.1098/rstb.2009.0267
Gabriel W. 1999. Evolution of reversible plastic responses: inducible defenses and environmental tolerance, pp. 286–305. In: Tollrian R. & Harvell D. (eds), The Ecology and Evolution of Inducible Defenses, Princeton University Press, Princeton, New Jersey, 395 pp. ISBN: 9780691004945
Gómez V.I. 2012. Respuestas fenotípicas en larvas de anuros a situaciones cambiantes en los factores bióticos y abióticos. Tesis Doctoral, Universidad Nacional de La Plata, Facultad de Ciencias Naturales Museo, 194 pp.
Gómez V.I. & Kehr A.I. 2011a. Morphological and developmental responses of anuran larvae (Physalaemus albonotatus) to chemical cues from the predators Moenkhausia dichoroura (Characiformes: Characidae) and Belostoma elongatum (Hemiptera: Belostomatidae). Zool. Stud. 50(2): 203–210.
Gómez V.I. & Kehr A.I. 2011b. Morphological and developmental plasticity in larvae of Physalaemus santafecinus (Anura: Leiuperidae) in response to chemical cues of different predators. Phyllomedusa: J. Herpetol. 10(2): 143–151. DOI: http://dx.doi.org/10.11606/issn.2316-9079.v10i2p143-151
Gómez V.I. & Kehr A.I. 2012. Effects of predator fish and water bugs on morphology and development of tadpoles of Elachistocleis bicolor (Anura: Microhylidae). Biologia 67(5): 1001–1006. DOI: 10.2478/s11756-012-0082-1
Gosner K.L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16(3): 183–190.
Griffiths R. A. 1991. Competition between common frog, Rana temporaria, and natterjack toad, Bufo calamita, tadpoles: the effect of competitor density and interaction level on tadpole development. Oikos 61(2): 187–196.
Jara F.G. & Perotti M.G. 2009. Tadpoles responses to predation risk. Ontogenetic change between constitutive and inducible defenses. J. Herpetol. 43(1): 82–88. DOI: http://dx.doi.org/10.1670/07-229R2.1
Kehr A.I. 1989. Factores dependientes de la densidad y su influencia sobre el crecimiento individual de los estados larvales de Hyla pulchella pulchella (Amphibia:Anura). Limnobios 2: 757–761.
Kehr A.I. 1994. Patrones de dispersión espacio-temporales y su influencia en la biología larval de Bufo arenarum (Amphibia: Anura). Neotrópica 40: 35–40.
Kehr A.I. 1997. Stage-frequency and habitat selection of a cohort of Pseudacris ocularis tadpoles (Hylidae: Anura) in a Florida temporary pond. Herpetol. J. 7(3): 103–109.
Kehr A.I. & Marangoni F. 1999. Variación intrapoblacional en el crecimiento y tamańo en la metamorfosis de Bufo paracnemis, bajo condiciones naturales. Neotrópica 45: 63–68.
Kingsolver J.G. 1995. Fitness consequences of seasonal polyphenism in western white butterflies. Evolution 49(5): 942–954
Loman J. 2004. Density regulation in tadpoles of Rana temporaria: a full pond field experiment. Ecology 85(6): 1611–1618.
Maciel T.A. & Juncá F.A. 2009. Effects of temperature and volume of water on the growth and development of tadpoles of Pleurderma diplolister and Rhinella granulosa (Amphibia: Anura). Zoologia 26(3): 413–418.
Newman R.A. 1988. Adaptive plasticity in development of Scaphiopus couchii tadpoles in desert ponds. Evolution 42(4): 774–783.
Peacor S.D. 2002. Positive effects of predators on prey growth rate through induced modifications of prey behavior. Ecol. Lett. 5(1): 77–85. DOI: 10.1046/j.1461-0248.2002.00287.x
Petranka J.W. & Hayes L. 1998. Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav. Ecol. Soc. 42(4): 263–271. DOI: 10.1007/s002650050438
Pfennig D.W., Mabry A. & Orange D. 1991. Environmental causes of correlations between age and size at metamorphosis in Scaphiopus multiplicatus. Ecology 72: 2240–2248. DOI: http://dx.doi.org/10.2307/1941574
Relyea R.A. 2002. Competitor-induced plasticity in tadpoles: consequences, cues, and connections to predators induced plasticity. Ecol. Monogr. 72(4): 523–540. DOI: 10.2307/3100055
Relyea R.A. 2003. How prey respond to combined predators: a review and an empirical test. Ecology 84(7): 1827–1839. DOI: http://dx.doi.org/10.1890/0012-9658(2003)084[1827:HPRTCP]2.0.CO;2
Relyea R.A. 2004. Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology 85: 172–179. DOI: http://dx.doi.org/10.1890/03-0169
Relyea R.A. 2007. Getting out alive: how predators affect the decision to metamorphose. Oecologia 152(3): 389–400. DOI: 10.1007/s00442-007-0675-5
Relyea R.A. & Werner E.E. 1999. Quantifying the relation between predator-induced behavioral responses and growth performance in larval anurans. Ecology 80(6): 2117–2124. DOI: http://dx.doi.org/10.1890/0012-9658(1999)080[2117:QTRBPI]2.0.CO;2
Reques R. & Tejedo M. 1995. Negative correlation between length of larval period and metamorphic size in natural populations in natural populations of natterjack toads (Bufo calamita). J. Herpetol. 29: 311–314.
Richter-Boix A., Tejedo M. & Rezende E. 2011. Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecol. Evol. 1(1): 15–25. DOI: 10.1002/ece3.2
Skelly D.K. 1995. Competition and the distribution of spring peeper larvae. Oecologia 103(2): 203–207. DOI: 10.1007/BF00329081
Tejedo M. & Reques R. 1992. Effects of egg size and density on metamorphic traits in tadpoles of the natterjack toad (Bufo calamita). J. Herpetol. 26: 146–152.
Tejedo M. & Reques R. 1994. Does larval growth history determine timing of metamorphosis in anurans? A field experiment. Herpetologica 50(2): 113–118.
Teplitsky C. & Laurila A. 2007. Flexible defense strategies: competition modifies investment in behavioral vs. morphological defenses. Ecology 88(7): 1641–1646. PMID: 17645010
Tollrian R. & Harvell D. (eds) 1999. The Ecology and Evolution of Inducible Defences. Princeton University Press, Princeton, New Jersey, 395 pp. ISBN: 9780691004945
Travis J. 1984. Anuran size at metamorphosis: Experimental test of a model based on intraspecific competition. Ecology 65: 1155–1160. DOI: http://dx.doi.org/10.2307/1938323
Van Buskirk J. & Relyea R.A. 1998. Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol. J. Linn. Soc. 65(3): 301–328. DOI: 10.1111/j.1095-8312.1998.tb01144.x
Warner S.C., Dunson W.A. & Travis J. 1991. Interaction of pH, density, and priority effects on the survivorship and growth of two species of hylid tadpoles. Oecologia 88(3): 331–339. DOI: 10.1007/BF00317575
Wassersug R.J. 1975. The adaptive significance of the tadpole stage with comments on the maintenance of complex life cycles in anurans. Am. Zool. 15(2): 405–417. DOI: 10.1093/icb/15.2.405
Werner E.E. & Peacor S.D. 2003. A review of trait-mediated indirect interactions in ecological communities. Ecology 84(5): 1083–1100. DOI: http://dx.doi.org/10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2
West-Eberhard M.J. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20: 249–278. DOI: 10.1146/annurev.es.20.110189.001341
Wilbur H.M. 1980. Complex life cycles. Annu. Rev. Ecol. Syst. 11: 67–93. DOI: 10.1146/annurev.es.11.110180.000435
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kehr, A.I., Gómez, V.I. Influence of density and predators on metamorphic size in Rhinella schneideri tadpoles raised in mesocosm conditions. Biologia 69, 1417–1424 (2014). https://doi.org/10.2478/s11756-014-0438-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-014-0438-9