Abstract
Adsorption of 90Sr on hydroxyapatite is important in many fields of life. The aim of the paper was to discuss the changes in the double electrical layer at the hydroxyapatite/electrolyte solution interface during Sr ions adsorption and to establish the relations between time and adsorption on this material. Hydroxyapatite was prepared by the wet method, characterized by the X-ray diffraction and accelerated surface area and porosimetry methods. Studies of adsorption were carried out using 90Sr isotopes and ionic solutions containing this ion depending on concentration and pH of the solution. Sr adsorption isotherms on hydroxyapatite were determined and the effect of strontium ions presence on the surface charge density and zeta potential was discussed.
Similar content being viewed by others
1 Introduction
Hydroxyapatite Ca10(PO4)6(OH)2-(HAP), is a mineral present in igneous and metamorphic rocks, sediments, soil and, suspended particles (Corbridge 1985). It is also a major inorganic constituent of bones and teeth (Narasaraju and Phebe 1996). Its sorption properties are of great importance for environmental processes and various industrial purposes including fertilizer production, water purification and fabrication of biocompatible ceramics.
It has also been studied as a mineral sorbent for metal ions such as Pb2+ (Xu and Schwartz 1994; Ma et al. 1993), Cd2+ (Skwarek and Janusz 2015), Zn2+ (Skwarek 2014), Sr2+ (Lazic and Vukovi 1991), Al3+, Co2+, Cu2+, Fe2+, Ni2+ (Reichert and Binner 1996) and organic compounds (Fargues et al. 1998; Pinto et al. 2005). Since the sorption processes often occur in aqueous solutions it is very important to understand the hydroxyapatite behaviour; its surface properties and solubility in aqueous solutions under various conditions: pH, ionic strength, presence of Ca2+, PO4 3− and other ions in the solution, temperature, solid to solution ratio. In a study Dorozhkin (1997) discussed various models already proposed for apatite dissolution. It is evident that the experimental and theoretical results available in the literature were obtained under different experimental conditions and using different techniques. Hydroxyapatite solubility studies either show or suggest the presence of adsorbed ionic species, complexes or precipitates at the interface. As a consequence, the Ca:P ratio in the liquid phase is nonstoichiometric and dependent on the solid to solution ratio. The results of Dorozhkin’s nanolevel investigations have shown the best agreement between the congruent: incongruent theory and his new surface mechanism. Hydroxyapatite point of zero charge (pHPZC), as an important surface property, has also been studied (Janusz et al. 2008a).
Sorption of Sr2+ was investigated as it is a representative of alkaline earth cations and also because 90Sr (beta emitter, with t1/2 = 29.1 years), the important radioactive isotope in the environment behaves chemically similar to calcium and tends to concentrate in bones and teeth whose a principal inorganic constituent is hydroxyapatite (Lagett et al. 1982). Selective removal of radioisotopes from liquid nuclear wastes provides advantages like more waste volume reduction compared to the other methods such as chemical precipitation or direct solidification into concrete. Due to the stability towards high radiation, as well as high selectivity, the inorganic ion exchange sorbents are preferred over organic ion exchange resins in nuclear waste management facilities. Hydroxyapatite is a suitable sorbent for heavy metals and radionuclides due to its low water solubility, high stability under reducing and oxidizing conditions, and good buffering properties (Rosskopfova et al. 2012).
Strontium is an element abundant in natural. It is present in water and food. However, its stable isotopes are not considered as a toxic. Its biological role focused scientific interests especially in bones (Nielsen 2004; Dahl et al. 2001). The adsorption of strontium ions on the hydroxyapatite surface and hydroxyapatite or/and substitution of strontium ions hydroxyapatite crystal structure changing the hydroxyapatite properties such as solubility and bone mineral density (Christoffersen et al. 1997). Adsorption of Sr2+ ions at the synthetic hydroxyapatite was reported by I.D. (Smiciklas et al. 2000). However, their paper presents only the results of Sr ions adsorption from solution of the initial concentration 0.001 and 0.0001 mol/dm3 as the pH function.
In the present paper, we report more comprehensive studies of the kinetic and static adsorption of Sr2+ ions at the hydroxyapatite/electrolyte interface as the pH function pH for the initial concentration of Sr2+ ions in the range from 0.000001 to 0.001 mol/dm3. Adsorption measurements were complemented by the studies on surface charge density and the ζ potential determination. The effect of Sr2+ ions adsorption on the properties of the electrical double layer parameter is discussed.
2 Experimental
Hydroxyapatite was prepared by the wet method. Synthetic hydroxyapatite was prepared according to the reaction (Liu et al. 2003):
0.25 mol/dm3 (NH4)2HPO4 solution (analytically pure reagent produced by POCH) was added to 0.5 mol/dm3 Ca(NO3)2 solution prepared from Ca(NO3)2∙4H2O analytically pure, 1 dm3/h the reaction proceeded at the constant temperature at the constant pH equal to 12 pH checked by means of NH4OH with continuous stirring. After the addition of the whole amount of ammonium hydrogen phosphate, the solution was brought to boiling and left for another 10 min. The prepared sediment was left for a day, then washed and dried at 60–150 °C, next calcinated at 200–400 °C.
Hydroxyapatite was washed with doubly distilled water until the constant conductivity about 0.5 μS/cm was achieved. Surface charge measurements were performed simultaneously in the suspension of the same solid content, to keep the identical conditions of the experiments in a thermostated Teflon vessel at 25 °C. To eliminate the influence of CO2 all potentiometric measurements were performed under nitrogen atmosphere. pH values were measured using a set of glass REF 451 and calomel pHG201-8 electrodes with the Radiometer assembly. Surface charge density was calculated from the difference of the amounts of added acid or base to obtain the same pH value of suspension as for the background electrolyte. As a background electrolyte NaClO4 solution was used at concentrations 0.1, 0.01 and 0.001 mol/dm3. The adsorption was studied in the range of the initial concentration from 0.000001 to 0.001 mol/dm3 and as the function of pH. The adsorption of Sr ions was determined by the radiotracer method using radioisotopes. The radioactivity of electrolyte solution before and after adsorption was measured using the liquid scintillation counter LS5000D by Beckman. Based on the changes of radioactivity before and after adsorption, there was calculated density of ions sorption (Γ) on the hydroxyapatite surface according to the equations:
and:
where V is the solution volume (dm3), c0 is the initial concentration (mol/dm3), Sw is the specific surface area (m2/g), m is the weighed sediment over weighted (g), N0 is the number of counting from the source taken up before adsorption, Nr is the number of counting from the source taken up during titration, and cr is the equilibrium concentration (mol/dm3).
The zeta potential of hydroxyapatite dispersions were determined by the electrophoresis with Zetasizer 3000 by Malvern. The measurements were performed at 100 ppm solid concentration ultrasonication of the suspension. The particle size distributions were determined by means of the Mastertasizer 2000 apparatus produced by Malvern.
The specific surface area of the obtained sample was determined by the BET method. To determine parameters of sample surface porous structure, the low temperature nitrogen adsorption–desorption method was used. The measurements were made by means of the automatic adsorption analyzer of the ASAP 2405 type (Accelerated Surface Area and Porosimetry) by the Micromeritics Instruments, Co firm.
The crystalline structure was determined using the X-radiation diffraction. The XRD radiation diffraction was studied by means of a diffractometer equipped with a rtg generator of the ISO-DEBYFLEX 303–60 kV type produced by the Seifert Analytical X-ray firm and a cooling system KMW 3000C produced by the Oxford Diffraction firm. The measurement data were collected, analysed and processed using the XRAYAN program.
3 Results and discussion
The hydroxyapatite sample has the peaks characteristic of the crystalline hydroxyapatite form. This is evidenced by the peaks and their intensity: 25.75–40; 31.75–100; 34.15–25; 39.85–20; 46.85–30; 49.5–30 % which is in agreement with the phase analysis made from the ASTM base data. The hexagonal (point group P63/m) hydroxyapatite crystal structure contains two different cation sites, CaI and CaII, but one phosphate environment. Cations at the site CaI are bounded to nine oxygens belonging to six PO4 3− forming triangles and positioning in columns parallel to the OH– channels. Cations at the site CaII are surrounded by seven oxygen atoms belonging to five PO4 3− anions and one OH-anion. Cations smaller than Ca as well as low concentrations of slightly larger cations are preferably incorporated into the site CaI, where stronger interactions are present, while larger cations should be accommodated in the position CaII, even at high concentrations (Boanini et al. 2010). Sr2+ ions (ionic radius 0.12 nm) can replace Ca2+ ions (ionic radius 0.099 nm) in the structure of hydroxyapatite over the whole range of composition. Sr2+ ions are substituted preferentially at the CaII site, but at low concentration they are preferably accommodated the in site CaI.
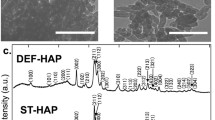
The XRD spectrum of prepared sample of hydroxyapatite and sample with adsorbed Sr2+ ions form solution of initial concentration 0.001 mol/dm3 are depicted in Fig. 1. Analysis of presented data indicated that the adsorption of strontium at hydroxyapatite sample does not changes unit cell that are a = 0.943 nm c = 0.688 nm in for both samples. But the crystal size is different, for samples before adsorption of strontium was 43.2 nm and after strontium adsorption decreases and was equal 13.2 nm. The decrease of crystal size upon strontium adsorption indicate that during the adsorption the dissolution and precipitation processes occur.
The Fig. 2 depicts the particle size distribution of the hydroxyapatite sample and hydroxyapatite with Sr2+ adsorbed from the solution with starting concentration 0.001 mol/dm3 of Sr ions. The particles diameter of the hydroxyapatite obtained falls between 0.35 and 12.6 μm (diameters characteristic for the distribution are d(0.1) = 0.456 μm, d(0.5) = 0.695 μm i d(0.9) = 1.516 μm. In the case of sample with Sr2+ adsorbed diameters falls between 0.5 and 20 μm (diameters characteristic for the distribution are d(0.1) = 0.596 μm, d(0.5) = 0.938 μm i d(0.9) = 1.908 μm). It is visible that strontium ions adsorption changes the particle size distribution of the hydroxyapatite causing that small particles disappear and bigger ones emerge. This results confirms processes of dissolution and precipitation of the hydroxyapatite in strontium ions solution. Additional confirmation of this effect are measurements of specific surface and porosity of samples before and after strontium adsorption listed in the Table 1. It is visible that Sr2+ ions adsorption causes specific surface drop and increase of pores diameter.
The point of zero charge (pHPZC) was determined by potentiometric titration with 0.001 mol/dm3 NaClO4 as an background electrolyte.
The former represents the pH value of the solution where the net surface charge is zero, and the latter is the pH value of the solution where electrokinetic potential of the solid particles is equal to zero. Equality between pH values at pHPZC and at pHIEP exists only if no specific sorption of ions from the solutions occurs. pHPZC can be determined by the potentiometric titration and batch equilibration technique, while the methods for pHIEP determination are electrophoresis, electroosmosis, measurements of streaming potential, etc. (Milonjicet et al. 1983). On the other hand, Na+ salts are not indifferent because ion exchange between Na+ ions from the solution and Ca2+ ions from the solid phase take place (Bell et al. 1973). The specific sorption of Na+ shifts pHPZC to lower values. The pHPZC and pHIEP values differ significantly and it is very difficult to compare them. The main reason for this is the fact that all experimental results were obtained for the hydroxyapatite samples of different origin, stoichiometry and purity, under various experimental conditions, and by different experimental techniques. Since the hydroxyapatite surface consists of both proton-bearing and salt type functional groups, the calcium and phosphate ions are also the potential determining ones. Adsorption of a large amount of Ca2+, PO4 3−, OH− or CO3 2− on hydroxyapatite may occur during the preparation of hydroxyapatite samples, so the desorption of these ions may cause an alteration in equilibrium pH. According to Wu et al. (1991), the main reactions for the surface properties of hydroxyapatite in aqueous solution are:
In the case of lower initial pH values (i.e., below pHpzc) of the solution, the consumption of protons from the same surface are by the protonation of surface =PO- and =CaOH0 groups which results in a final increase in pH. As a result, the positively charged =CaOH2 + and the neutral =POH0 sites will prevail on the hydroxyapatite surface in acidic solutions, making the surface charge of hydroxyapatite positive in this pH region. On the other hand, final pH decrease takes place in the range of higher initial pH (i.e., above pHpzc) due to OH− consumption via deprotonation of surface =CaOH2 + and =POH0 sites. Hence, the neutral =CaOH0 and the negatively charged =PO− species predominate in alkaline solutions, resulting the hydroxyapatite surface to become negatively charged in alkaline solutions. The pHpzc and pHIEP values (Table 1) obtained for each system hydroxyapatite/NaClO4 are consistent with the literature (Janusz et al. 2008a, b).
The pHPZC dependence on the solid/liquid ratio could be related with hydroxyapatite incongruent solubility (Smicˇiklas et al. 2000). Dimovic et al. (2009) presented that the pHPZC value of powered bone sorbents annealed at 800 and 1000 °C was 10.15 and 10.00, respectively. The XRD patterns of the powders were similar to those of well-crystallized synthetic hydroxyapatite. It could be supposed that in the case of the hydroxyapatite prepared by calcination, the shift of pHPZC could be higher according to the used sintering temperature.
The mechanisms of the metal cations retention are different and include ionic exchange reactions at the surface or co-precipitation of low soluble metal phosphate phases. Hydroxyapatite could provide phosphates by dissolution in the acidic environment. These phosphates can react with metal ions to precipitate low soluble metal phosphate crystals with an apatitic structure according to:
These phosphates can combine with Sr2+ ions precipitating sparsely soluble crystals of Sr2+ phosphates of the apatite structure according to the equation:
According to the second theory of ionic exchange, metal ions which are adsorbed on the surface of apatite molecules, will replace Ca2+ ions from the hydroxyapatite lattice in the diffusion process:
There is a large difference between the pHpzc i pHIEP values for the hydroxyapatite/NaClO4 system. At pH <4 particle charge will be affected mainly by phosphate groups. If the studied system was left for a few days, pHIEP value might increase due to specific sorption of Na ions on the hydroxyapatite surface and crashing away of phosphate ions from it but if it reached the pHpzc value. The increase in the pHpzc and pHIEP values in the hydroxyapatite/NaClO4/Sr system is due to the effect of positive charge caused by the presence of Sr2+ ions.
3.1 Adsorption of Sr2+ ions
Sr2+ ions belong to those which occur in the non-hydrolized form in aqueous solutions, do not form sparsely soluble oxides or hydroxides. With OH− ions they form the one ligand complex:
Figure 3 presents the fraction of Sr2+ ionic forms in aqueous solutions calculated based on reaction. As can be seen up to pH 10.5 Sr2+ is a predominant forms, contribution of the SrOH+ form is significant only in alkaline solutions. Thus Sr2+ ions is convenient in the studies of specific adsorption with ion. This is confirmed by the XRD studies where formation of a new phase after strontium adsorption on hydroxyapatite was not observed. The value pH 10.5 excludes deposition of strontium hydroxide on the hydroxyapatite surface which would result in formation of a new compound based on hydroxyapatite. Adsorption of metal cations on surface hydroxyl groups usually proceeds according to the reactions:
According to the above reactions cations adsorption leads to release of H+ ions and on the surface there are formed combinations of complex character in which: in the case of extra spherical complexes the cation occupies a position in IHP and in the case of inner-sphere complexes the cation occupies the position close the adsorbent surface. Both reactions presented above lead to the increase of concentration of negatively charged groups on the solid surface which results in drop of surface charge density. However, this effect was not observed for the studied systems. The plane passing through the centres of ions forming inner-sphere complexes is situated much closer to the surface than the IHP plane. However, according to same reports the plane is in the same place as the surface one. Density of inner-sphere complexes adsorption affects the ζ potential as the charge density in the compact electrical double layer changes.
The pH of a solution is an important parameter that controls adsorption process because of ionization of surface functional groups and alteration of the solution composition. Sorption of Sr2+ on the hydroxyapatite was studied using the pH from 7 to 11. The composition of the surface phase of hydroxyapatite is constant in solution of pH in the range 4–10 (Bengston et al. 2009). Since hydroxyapatite is the salt of slight acid, it could run over two-step hydrolysis in an aqueous solution on the hydroxyapatite surface. The amount of Ca2+ ions in solution depends on the stage, which dominates under existing conditions. In the range of the lower initial pH values, the consumption of protons from the solution by the protonation of negatively charged: =PO− (‘‘=’’ the surface) and neutral: =CaOH0 surface groups results in a shift of final pH to higher values. The positively charged: =CaOH2 + species and neutral: =POH0 sites prevail in acidic solutions. Due to the high pH value, the surface of hydroxyapatite is deprotonated, releasing H+ ions in the solution and causing a shift of pH to lower value. The negatively charged: =PO− sites and neutral =CaOH0 sites predominate in alkaline solutions (Smicˇiklas et al. 2006).
Dependence of density of Sr2+ ions adsorption on pH is presented in Figs. 4 and 5 for the concentrations 0.0000001 and 0.001 mol/dm3 of Sr2+ ions respectively. As can seen for both studied initial concentrations, adsorption of Sr2+ ions in the pH function increases. Such course of adsorption is similar to the reaction of cation exchange with H+ ions of surface hydrogen oxygen group in the metal oxide and electrolyte solution systems. As hydroxyapatite dissolves in acidic solutions, concentration of Ca2+ ions increases with the decreasing pH value (Chena et al. 2004).
Adsorption of Sr2+ ions on the hydroxyapatite surface can proceed through the exchange of Ca2+ ions according to the reaction:
A characteristic feature of strontium ions adsorption on hydroxyapatite is a relatively slow decrease of Sr ions concentration from the solution due to adsorption e.g. at the initial concentration of Sr2+ ions 0.000001 mol/dm3 at pH 7.6, the loss is 64 % and increases with the increasing pH reaching about 93 % at pH 10.7. Studying Sr ions adsorption on hydroxyapatite no multivalent cations were observed on metal oxides of adsorption edges which are connected with the exchange of metal cation with proton in the surface hydroxyl group of metal oxide (Janusz et al. 2004).
The dependence of Sr2+ ions adsorption on the hydroxyapatite sample in the function of equilibrium concentration of strontium ions is presented in Fig. 6 in the form of log adsorption—log concentration. As can be seen for three initial concentrations, the log adsorption dependence in the log concentration function is rectilinear (the correlation coefficient equals 0.999). As follows from the data presented in Fig. 5, adsorption of Sr2+ ions can be described by the Freundlich equation (Stumm 1992) which describes adsorption on the surface with heterogeneous adsorption sites.
The dependence of surface charge density on pH for the hydroxyapatite/0.001 NaClO4 mol/dm3 solution system in the presence of Sr2+ ions is presented in Fig. 7. As can be seen Sr2+ ions adsorption has an insignificant effect on the amount of H+ ions released from the surface. The results confirm that Sr2+ adsorption on hydroxyapatite does not proceed as in the case of multivalent cations adsorption the metal oxide surface where, due to H+ ions release, a negative charge is created through the exchange of Ca2+ ions from the crystalline form of hydroxyapatite. The dependence of dzeta potential on pH for the hydroxyapatite/NaClO4 solution system in the presence of strontium ions is presented in Fig. 8. The dzeta potential value increases with the increasing strontium concentration. This results from Sr2+ ions adsorption in the compact part of double electrical layer and overcharge of this area. The presence of Sr2+ ions of the highest initial concentrations in the hydroxyapatite/electrolyte systems causes overcharge of the compact double electrical layer and appearance of CR2 point at pH 7 and 8.2 for the 0.001 and 0.0001 mol/dm3 strontium ion solutions respectively.
3.2 Kinetics of Sr2+ adsorption
Adsorption rate depends on many factors which are: size and structure of adsorbed molecules, kind of solvent and porous structure of adsorbent (Ościk 1983). The adsorption process includes two stages: transport of adsorbate to the adsorbent surface as well as adsorption on the surface. The first stage is governed by the diffusion laws and depends on the kind of solvent and adsorbate. The adsorption process is very fast and it is difficult to determine the time of its course.
There are so many models which can be used to explain the kinetics of sorption processes (e.g. first-order, pseudo-first order, and pseudo-second-order reaction models). The studies on the kinetics of the various sorption processes have revealed that over a long period of time the pseudosecond-order model gives the best correlation (Ho and McKay 1999).
At the interface during the Sr2+ ions adsorption, ion exchange, adsorption and hydroxyapatite dissolution can proceed. Changes of Sr2+ ions concentration and pH in the time function are presented in Figs. 9 and 10.
Kinetics of pH and Sr2+ ions adsorption changes on hydroxyapatite from the Sr2+ ions solution of the initial concentration 0.001 mol/dm3. Points indicate the experimental data, the broken line-the pseudo first order model for qe = 2.244 and k1 = 1.610, the broken line of longer segments-the pseudo second order model for qe = 2.259 and k2 = 2999. The continuous line-multi exponential model: aeq = 7029, A0 = 0.767, k1 = 2.526, A1 = 0.066, k2 = 0.0009, A2 = 0.167
Kinetics of pH and Sr2+ ions adsorption changes on hydroxyapatite from the Sr2+ ions solution of the initial concentration 0.0001 mol/dm3. Point indicate the experimental data, the broken line-the pseudo first order model for qe = 0.197 and k1 = 1.672, the broken line of longer segments-the pseudo second order model for qe = 0.199 and k2 = 27,860. The continuous line-multi exponential model: aeq = 4761, A0 = 0.856, k1 = 2.023, A1 = 0.0056, k2 = 0.00001, A2 = 0.138
The change of concentration in the logarithm function has a complex course in the first minutes after the addition of hydroxyapatite to the Sr2+ ions solution. A fast adsorption process which, in the case of low initial concentrations of Sr2+ ions (0.0001 mol/dm3), leads to equilibrium is observed. Kinetics of Sr2+ ions adsorption presented in Fig. 9 can be well described by the pseudo-first order equation. In the case of the initial concentration 0.001 mol/dm3 of Sr2+ ions after fast adsorption its slow stage connected with diffusion in microchannels of crystalline line of hydroxyapatite is observed (Madhavi et al. 2005). In this case the adsorption kinetics is best described by the multi exponential equation (Fig. 10).
4 Conclusion
The study of the Sr ions adsorption at the hydroxyapatite/electrolyte solution interface and the changes of the electrical double layer (edl) structure in this system are presented. The results of measurements of Sr ions adsorption kinetics on hydroxyapatite were fit to the adsorption models and the best were chosen for the process description. The adsorption of Sr, ions at hydroxyapatite contributed to the increase of the zeta potential as well as that change of the double electrical layer structure. The characteristic points for the double electrical layer for the compound prepared according are pHPZC = 7.5 i pHIEP < 4 but after the Sr2+ adsorption pHPZC = 9.7 pHIEP = 6 The presence of Sr2+ ions in the solutions of initial concentration results in overloading of the compact double electrical layer and appearance of the point CR2. Adsorption of Sr ions changes in the studied pH range and can be described using the Freundlich equation, which describes adsorption on the surface with heterogeneous adsorption sites. As can be seen Sr2+ ions adsorption has an insignificant effect on the amount of H+ ions released from the surface. The decrease of crystal size upon strontium adsorption indicate that during the adsorption the dissolution and precipitation processes occur. It is visible that strontium ions adsorption changes the grain distribution of the hydroxyapatite causing that small particles disappear and bigger ones emerge. This results confirms processes of dissolution and precipitation of the hydroxyapatite in strontium ions solution. The adsorption kinetics is best described by the multiexponential equation.
Generally, cation removal from aqueous solutions by hydroxyapatite can occur through different sorption processes adsorption, ion-exchange, surface complexation, co-precipitation, recrystallization, depending on the experimental conditions and nature of both sorbing cations and hydroxyapatite itself. At the pH values higher than pHPZC, the sorbent surface becomes negative, due to adsorption of OH− from the solution. Increase in the electrostatic attraction forces, acting between the surface and the cations from the solution, contributes to a greater cation sorption at higher pH. The observed good adsorption properties of hydroxyapatite are particularly important for its possible practical application treatment of wastewaters from chemical processing.
References
Bell, L.C., Posner, A.M., Quirk, J.P.: The point of zero charge of hydroxyapatite and fluorapatite in aqueous solutions. J. Colloid Interface Sci. 42, 250–261 (1973)
Bengston, A., Shuchukarev, A., Persson, P., Sjo¨berg, S.: A solubility and surface complexation study of a non-stoichiometric hydroxyapatite. Geochim. Cosmochim. Acta 73, 257–267 (2009)
Boanini, E., Gazzano, M., Bigi, A.: Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 6, 1882–1894 (2010)
Christoffersen, J., Christoffersen, M.R., Kolthoff, N., Bärenholdt, O.: Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone 20, 47–54 (1997)
Corbridge, D.E.C.: Phosphorus: An Outline of its Chemistry, Biochemistry and Technology. Elsevier, Amsterdam (1985)
Chena, Z.-F., Darvellb, B.W., Leung, V.W.-H.: Hydroxyapatite solubility in simple inorganic solutions. Arch. Oral Biol. 49, 359–367 (2004)
Dimovic´, S., Smicˇiklas, I., Plec´asˇ, I., Antonovic´, D., Mitric´, M.: Comparative study of differently treated animal bones for Co2+ removal. J. Hazard Mater. 164, 279–287 (2009)
Dahl, S.G., Allain, P., Marie, P.J., Mauras, Y., Boivin, G., Ammann, P., Tsouderos, Y., Delmas, P.D., Christiansen, C.: Incorporation and distribution of strontium in bone. Bone 28, 446–453 (2001)
De SouzaFigueiredo, K.C., MartinsSalim, V.M., Alves, T.L.M., Pinto, J.C.: Lysozyme adsorption onto different supports: a comparative study. Adsorption 11(2), 131–138 (2005)
Dorozhkin, S.V.: Surface reactions of apatite dissolution. J. Colloid Interface Sci. 191, 489–497 (1997)
Fargues, C., Bailly, M., Grevillot, G.: Adsorption of BSA and hemoglobin on hydroxyapatite support: equilibria and multicomponent dynamic adsorption. Adsorption 4(1), 5–16 (1998)
Ho, Y.S., McKay, G.: Pseudo-second order model for sorption processes. Process. Biochem. 34, 451–465 (1999)
Janusz, W., Gałgan, A., Skwarek, E.: Effects of the adsorption of Ba2+ ions on the electrical double layer at the 4th group metal oxide/electrolyte interface. Adsorpt. Sci. Technol. 22, 795–806 (2004)
Janusz, W., Skwarek, E., Pasieczna-Patkowska, S., Slósarczyk, A., Paszkiewicz, Z., Rapacz-Kmita, A.: FT-IR/PAS investigations of the electrical double layer at the calcium phosphate/solution interface. Eur. Phys. J. 154, 329–333 (2008a)
Janusz, W., Skwarek, E., Złotucha, A., Reszka, M.: The study of the acid–base properties of the surface groups at the hydroxyapatite/NaClO4 solution interface. Pol. J. Chem. 82, 57–67 (2008b)
Lagett, R.W., Eckerman, K.F., Williams, L.R.: Strontium-90 in bone: a case study in age-dependent dosimetric modelling. Health Phys. 43, 307–322 (1982)
Lazic, S., Vukovi, Z.: Ion exchange of strontium on synthetic hydroxyapatite. J. Radioanal. Nucl. Chem. Artic. 149, 161–168 (1991)
Liu, J., Ye, X., Wang, H., Zhu, M., Wang, B., Yan, H.: The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 29, 629–633 (2003)
Ma, Q.Y., Traina, S.J., Logan, T.J.: In situ lead immobilization by apatite. Environ. Sci. Technol. 27, 1803–1810 (1993)
Madhavi, S., Ferraris, C., White, T.J.: Synthesis and crystallization of macroporous hydroxyapatite. J. Solid State Chem. 178, 2838–2845 (2005)
Milonjic, S.K., Ilic, Z.E., Kopec´ni, M.M.: Sorption of alkali cations at the zirconium oxide/aqueous electrolyte interface. Colloid Surf. 6, 167–174 (1983)
Narasaraju, T.S.B., Phebe, D.E.: Some physico-chemical aspects of hydroxyapatite. J. Mater. Sci. 31, 1–21 (1996)
Nielsen, S.P.: The biological role of strontium. Bone 35, 583–588 (2004)
Ościk, J.: Adsorpcja. PWN, Warszawa (1983)
Reichert, J., Binner, J.G.P.: An evaluation of hydroxyapatite-based filters for removal of heavy metal ions from aqueous solutions. J. Mater. Sci. 31, 1231–1241 (1996)
Rosskopfova´, O., Galambosˇ, M., Ometa´kova´, J., Cˇaplovicˇova´, M., Raje, C.P.: Study of sorption processes of copper on synthetic hydroxyapatite. J. Radioanal. Nucl. Chem. 293, 641–647 (2012)
Skwarek, E., Janusz, W.: Adsorption of Cd (II) ions at the hydroxyapatite/electrolyte solution interface. Sep. Sci. Technol. (2015). doi:10.1080/01496395.2015.1085878
Skwarek, E.: Adsorption of Zn on synthetic hydroxyapatite from aqueous solution. Sep. Sci. Technol. 49(11), 1654–1662 (2014)
Smicˇiklas, I.D., Milonjic´, S.K., Pfendt, P., Raicˇevic, S.: The point of zero charge and sorption of cadmium (II) and strontium (II) ions on synthetic hydroxyapatite. Sep. Purif. Technol. 18, 185–194 (2000)
Smicˇiklas, I., Dimovic, S., Plec´as, I., Mitric, M.: Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res. 40, 2267–2274 (2006)
Stumm, W.: Chemistry of the Solid-Water Interface, p. 93. Wiley, New York (1992)
Wu, L., Forsling, W., Schindler, P.W.: Surface complexation of calcium minerals in aqueous solutions. 1: surface protonation of fluoroapatite water interfaces. J. Colloid Interface Sci. 147, 178–185 (1991)
Xu, Y., Schwartz, F.W.: Lead immobilization by hydroxyapatite in aqueous solutions. J. Contam. Hydrol. 15, 187–206 (1994)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Janusz, W., Skwarek, E. Study of sorption processes of strontium on the synthetic hydroxyapatite. Adsorption 22, 697–706 (2016). https://doi.org/10.1007/s10450-016-9761-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-016-9761-5