Abstract
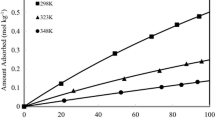
Adsorption isotherms of H2S, CO2, and CH4 on the Si-CHA zeolite were measured over pressure range of 0–190 kPa and temperatures of 298, 323, and 348 K. Acid gases adsorption isotherms on this type of zeolite are reported for the first time. The isotherms follow a typical Type-I shape according to the Brunauer classification. Both Langmuir and Toth isotherms describe well the adsorption isotherms of methane and acid gases over the experimental conditions tested. At room temperature and pressure of 100 kPa, the amount of CO2 adsorption for Si-CHA zeolite is 29 % greater than that reported elsewhere (van den Bergh et al. J Mem Sci 316:35–45 (2008); Surf Sci Catal 170:1021–1027 (2007)) for the pure silica DD3R zeolite while the amounts of CH4 adsorption are reasonably the same. Si-CHA zeolite showed high ideal selectivities for acid gases over methane at 100 kPa (6.15 for H2S and 4.06 for CO2 at 298 K). Furthermore, H2S adsorption mechanism was found to be physical, and hence, Si-CHA can be utilized in pressure swing adsorption processes. Due to higher amount of carbon dioxide adsorbed and lower heats of adsorption as well as three dimensional channels of Si-CHA pore structure, this zeolite can remove acid gases from methane in a kinetic based process such as zeolite membrane.









Similar content being viewed by others
Abbreviations
- b:
-
Affinity parameter of Langmuir or Toth isotherm (kPa−1)
- b∞ :
-
Affinity parameter of Langmuir or Toth isotherm at infinite temperature (kPa−1)
- C μs :
-
Saturation adsorption capacity of adsorbent (mol kg−1)
- C μ :
-
Amount adsorbed (mol kg−1)
- p:
-
Pressure (kPa)
- Qst :
-
Isosteric heat of adsorption (kJ mol−1)
- Q:
-
Isosteric heat of adsorption for Langmuir isotherm in Eq. 3 (kJ mol−1)
- R:
-
Universal gas constant (kJ K−1 mol−1)
- r:
-
Correlation coefficient
- T:
-
Temperature (K)
- T0 :
-
Reference temperature (K)
- t:
-
Heterogeneity parameter in Toth isotherm
- t0 :
-
Heterogeneity parameter at the reference temperature (T0)
- θ :
-
Fractional amount adsorbed
- α :
-
Adjustable parameter in Eq. 4
References
Ackley, M.W., Giese, R.F., Yang, R.T.: Clinoptilolite—untapped potential for kinetic gas separations. Zeolites 12, 780–788 (1992)
Ackley, M.W., Rege, S.U., Saxena, H.: Application of Natural Zeolites in the Purification and Separation of Gases, pp. 25–42. Elsevier Science, New York (2003)
Bae, Y.-S., Farha, O.K., Hupp, J.T., Snurr, R.Q.: Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification. J. Mater. Chem. 19, 2131–2134 (2009)
Bobonich, F.M.: Effect of chemical modification of clinoptilolite on the adsorption energy of dipolar and quadrupolar molecules. Theor. Exp. Chem. 31, 268–271 (1995)
Bolhàr-Nordenkampf, M., Friedl, A., Koss, U., Tork, T.: Modelling selective H2S absorption and desorption in an aqueous MDEA-solution using a rate-based non-equilibrium approach. Chem. Eng. Process 43(6), 701–715 (2004)
Bourrelly, S., Maurin, G., Llewellyn, P.L.: Adsorption microcalorimetry of methane and carbon dioxide on various zeolites. Stud. Surf. Sci. Catal. 158, 1121–1128 (2005)
Bullin, J.A., Polasek, J.C.: Selective Absorption Using Amines. In: Annual Convention Proceedings-Gas Process Association, Dallas, March 15–17, pp. 86–90 (1982)
Camblor, M.A., Corma, A., Lightfoot, P., Villaescusa, L.A., Wright, P.A.: Synthesis and structure of ITQ-3, the first pure silica polymorph with a two-dimensional system of straight eight-ring channels. Angew. Chem., Int. Ed. Engl. 36, 2659–2661 (1997)
Camblor, M.A., Villaescusa, L.A., Díaz-Cabañas, M.J.: Synthesis of all-silica and high-silica molecular sieves in fluoride media. Top. Catal. 9(1–2), 59–76 (1999)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095–1101 (2004)
Chue, K.T., Kim, J.N., Yoo, Y.J., Cho, S.H., Yang, R.T.: Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind. Chem. Eng. Res. 34, 591–598 (1995)
Crespo, D., Qi, G., Wang, Y., Yang, F.H., Yang, R.T.: Superior sorbent for natural gas desulfurization. Ind. Eng. Chem. Res. 47, 1238–1244 (2008)
Cruz, A.J., Pires, J., Carvalho, A.P., De Carvalho, M.B.: Physical adsorption of H2S related to the conservation of works of art: the role of the pore structure at low relative pressure. Adsorption 11(5–6), 569–576 (2005)
Daviet, G.R., Sundermann, R., Donnelly, S.T., Bullin, J.A.: Switch to MDEA raises capacity. Hydrocarbon Process 63, 79–82 (1984)
Delgado, J.A., Uguina, M.A., Gomez, J.M.: Adsorption equilibrium of carbon dioxide, methane and nitrogen onto mordenite at high pressures. Stud. Surf. Sci. Catal. 158, 1065–1072 (2005)
Den Exter, M. J.: Exploratory study of the synthesis and properties of 6-, 8- and 10-ring tectosilicates and their potential application in zeolite membranes. PhD Thesis, TU Delft Press, Delft, The Netherlands. Chap. 3–4 (1996)
Díaz-Cabañas, M.J., Barrett, P.A., Camblor, M.A.: Synthesis and structure of pure SiO2 chabazite: the SiO2 polymorph with the lowest framework density. Chem. Commun. 17, 1881–1882 (1998)
Dunne, J.A., Mariwala, R., Rao, M., Sircar, S., Gorte, R.J., Myers, A.L.: Calorimetric heats of adsorption and adsorption isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on silicalite. Langmuir 12(24), 5888–5895 (1996)
Duong, D.D.: Adsorption Analysis: Equilibria and Kinetics, p. 13. Imperial College Press, London (1998)
Ferino, I., Monaci, R., Rombi, E., Solinas, V., Burlamacchi, L.: Temperature-programmed desorption of H2S from alkali-metal zeolites. Thermochim. Acta 199, 45–55 (1992)
Fuderer, A., Rudelstorfer, E.: Selective adsorption processes. US Patent 3,896,849, 1976
Gabrielsen, J., Michelsen, M.L., Stenby, E.H., Kontogeorgis, G.M.: Modeling of CO2 absorber using an AMP solution. AIChE J. 52(10), 3443–3451 (2006)
Gaillard, M., Montouillout, V., Maugé, F., Fernandez, C.: An infrared and solid-state NMR study of the H2S adsorption on basic zeolite. Stud. Surf. Sci. Catal. 154, 1679–1685 (2004)
Garcia, C.L., Lercher, J.A.: Adsorption of H2S on ZSM-5 zeolites. J. Phys. Chem. 96, 2230–2235 (1992)
Graham, C., Pierrus, J., Raab, R.E.: Measurement of the electric quadrupole moments of CO2, CO and N2. Mol. Phys. 67, 939–955 (1989)
Herna′ndez-Maldonado, A.J., Yang, R.T., Chinn, D., Munson, C.L.: Partially calcined gismondine type silicoaluminophosphate SAPO-43: isopropylamine elimination and separation of carbon dioxide, hydrogen sulfide, and water. Langmuir 19, 2193–2200 (2003)
Himeno, S., Tomita, T., Suzuki, K., Yoshida, S.: Characterization and selectivity for methane and carbon dioxide adsorption on the all-silica DD3R zeolite. Microporous Mesoporous Mater. 98, 62–69 (2007a)
Himeno, S., Tomita, T., Suzuki, K., Nakayama, K., Yajima, K., Yoshida, S.: Synthesis and permeation properties of a DD3R-type zeolite membrane for separation of CO2/CH4 gaseous mixtures. Ind. Eng. Chem. Res. 46, 6989–6997 (2007b)
Hunt, H.K., Lew, C.M., Sun, M., Yan, Y., Davis, M.E.: Pure-silica zeolite thin films by vapor phase transport of fluoride for low-k applications. Microporous Mesoporous Mater. 128, 12–18 (2010a)
Hunt, H.K., Lew, C.M., Sun, M., Yan, Y., Davis, M.E.: Pure-silica LTA, CHA, STT, ITW, and –SVR thin films and powders for low-k applications. Microporous Mesoporous Mater. 130(1–3), 49–55 (2010b)
Ishibashi, M., Otake, K., Kanamori and S., Yasutake, A.: Study of CO2 removal technology from flue gas of thermal power plant by physical adsorption. In: Greenhouse Gas Control Technologies, Proceedings of the International Conference, pp. 95–100 (1999)
Jayaraman, A., Hernandez-Maldonado, A.J., Yang, R.T., Chinn, D., Munson, C.L., Mohr, D.H.: Clinoptilolites for nitrogen/methane separation. Chem. Eng. Sci. 59, 2407–2417 (2004)
Jensen, N.K., Rufford, T.E., Watson, G., Zhang, D.K., Chan, K.I., May, E.F.: Screening zeolites for gas separation applications involving methane, nitrogen, and carbon dioxide. J. Chem. Eng. Data 57(1), 106–113 (2012)
Kanezashi, M., O’Brien-Abraham, J., Lin, Y.S., Suzuki, K.: Gas permeation through DD3R-type zeolite membranes at high temperatures. AIChE J. 54, 1478–1486 (2008)
Karge, H.G., Raskó, J.: Hydrogen sulfide adsorption on faujasite-type zeolites with systematically varied Si-Al ratios. J. Colloid Interface Sci. 64, 522–532 (1987)
Keller, G.E., Anderson, R.A., Yon, C.M.: Adsorption. In: Rousseau, R.W. (ed.) Handbook of Separation Process Technology, pp. 644–696. Wiley Interscience, New York (1987)
Lide, D.R.: CRC Handbook of Chemistry and Physics, 89th edn. CRC Press/Taylor and Francis, Boca Raton (2009). (internet version)
Lin, Y.S., Kanezashi, M.: Gas permeation and diffusion in small and intermediate pore zeolite membranes. Stud. Surf. Sci. Catal. 170, 847–854 (2007)
Lutz, W., Suckow, M., Bülow, M.: Adsorption of hydrogen sulphide on molecular sieves. No enrichment in the presence of carbon dioxide. Gas Sep. Purif. 4, 190–196 (1990)
MacKenzie, H.D., Prambil, F.C., Daniels, C.A., Bullin, J.A.: Design & Operation of a selective sweetening plant using MDEA. Energy Prog. 7, 31–36 (1987)
Maurin, G., Bell, R., Kuchta, B., Poyet, T., Llewellyn, P.: Adsorption of non-polar and quadrupolar gases in siliceous Faujasite: molecular simulations and experiments. Adsorption 11, 331–336 (2005)
Nakayama, K., Suzuki, K., Yoshida, M., Yajima, K., Tomita, T.: Method for preparing DD3R type zeolite film, DD3R type zeolite film, and composite DD3R type zeolite film, and method for preparation thereof. US Patent App. 20,040,173,094, 2004
NIST Chemistry WebBook: http://webbook.nist.gov/chemistry/. Accessed Apr 2012
Olson, D. H.: Light hydrocarbon separation using 8-member ring zeolites. US Patent 6,488,741, 2002
Olson, D.H., Camblor, M.A., Villaescusa, L.A., Kuehl, G.H.: Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Microporous Mesoporous Mater. 67, 27–33 (2004)
Pacheco, M.A., Rochelle, G.T.: Rate-based modeling of reactive absorption of CO2 and H2S into aqueous methyldiethanolamine. Ind. Eng. Chem. Res. 37(10), 4107–4117 (1998)
Saha, D., Deng, S.G.: Adsorption equilibria and kinetics of carbon monoxide on zeolite 5A, 13X, MOF-5, and MOF-177. J. Chem. Eng. Data 54, 2245–2250 (2009)
Saha, D., Bao, Z., Jia, F., Deng, S.: Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 44, 1820–1826 (2010)
Sircar, S.: Separation of methane and carbon dioxide gas mixtures by pressure swing adsorption. Sep. Sci. Technol. 23, 519–529 (1988)
Sircar, S., Golden, T.C.: Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 35, 667–687 (2000)
Sircar, S., Kratz, W.C.: Simultaneous production of hydrogen and carbon dioxide from steam methane reformer off-gas by pressure swing adsorption. Sep. Sci. Technol. 23, 2397–2415 (1988)
Sircar, S., Kumar, R., Koch, W.R., VanSloun, J.: Recovery of methane from landfill gas. US Patent 4,770,676, 1988
Sircar, S.: Separation of multicomponent gas mixtures. US Patent 4,171,206, 1979
Tanada, S., Boki, K., Kita, T., Sakaguchi, K.: Adsorption behavior of hydrogen sulfide inside micropores of molecular sieve carbon 5A and molecular sieve zeolite 5A. Bull. Environ. Contam. Toxicol. 29, 624–629 (1982)
Tomita, T., Nakayama, K., Sakai, H.: Gas separation characteristics of DD3R type zeolite membrane. Microporous Mesoporous Mater. 68, 71–75 (2004)
van den Bergh, J., Zhu, W., Groen, J.C., Kapteijn, F., Moulijn, J.A., Yajima, K., Nakayama, K., Tomita, T., Yoshida, S.: Natural gas purification with a DD3R zeolite membrane: permeation modelling with Maxwell-Stefan equations. Stud. Surf. Sci. Catal. 170, 1021–1027 (2007)
van den Bergh, J., Zhu, W., Gascon, J., Moulijn, J.A., Kapteijn, F.: Separation and permeation characteristics of a DD3R zeolite membrane. J. Membr. Sci. 316, 35–45 (2008)
Vrachnos, A.: Thermodynamic modeling of acidic gas solubility in aqueous solutions of MEA, MDEA and MEA-MDEA blends. Ind. Eng. Chem. Res. 45, 5148–5154 (2006)
Xiang, Z., Peng, X., Cheng, X., Li, X., Cao, D.: CNT@Cu3(BTC)2 and metal-organic frameworks for separation of CO2/CH4 mixture. J. Phys. Chem. C 115(40), 19864–19871 (2011)
Yang, H., Gong, M., Chen, Y.: Preparation of activated carbons and their adsorption properties for greenhouse gases: CH4 and CO2. J. Nat. Gas Chem. 20(5), 460–464 (2011)
Yaşyerli, S., Ar, I., Doğu, G., Doğu, T.: Removal of hydrogen sulfide by clinoptilolite in a fixed bed adsorber. Chem. Eng. Process 41(9), 785–792 (2002)
Zhu, W., Kapteijn, F., Moulijn, J.A.: Shape selectivity in the adsorption of propane/propene on the all-silica DD3R. Chem. Commun. 24, 2453–2454 (1999)
Zhu, W., Kapteijn, F., Moulijn, J.A., Den Exter, M.C., Jansen, J.C.: Shape selectivity in adsorption on the all-silica DD3R. Langmuir 16, 3322–3329 (2000)
Acknowledgments
The authors are grateful for the experimental facilities for adsorption tests which were provided by the Gas Research Division of the Research Institute of the Petroleum Industries (RIPI). The financial support provided by the Gas Research Directorate of the National Iranian Gas Company is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maghsoudi, H., Soltanieh, M., Bozorgzadeh, H. et al. Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 19, 1045–1053 (2013). https://doi.org/10.1007/s10450-013-9528-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9528-1