Abstract
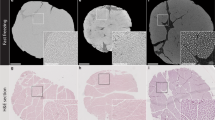
The outcome of both cryopreservation and cryosurgical freezing applications is influenced by the concentration and type of the cryoprotective agent (CPA) or the cryodestructive agent (i.e., the chemical adjuvants referred to here as CDA) added prior to freezing. It also depends on the amount and type of crystalline, amorphous and/or eutectic phases formed during freezing which can differentially affect viability. This work describes the use of X-ray computer tomography (CT) for non-invasive, indirect determination of the phase, solute concentration and temperature within biomaterials (CPA, CDA loaded solutions and tissues) by X-ray attenuation before and after freezing. Specifically, this work focuses on establishing the feasibility of CT (100–420 kV acceleration voltage) to accurately measure the concentration of glycerol or salt as model CPA and CDAs in unfrozen solutions and tissues at 20°C, or the phase in frozen solutions and tissue systems at −78.5 and −196°C. The solutions are composed of water with physiological concentrations of NaCl (0.88% wt/wt) and DMEM (Dulbecco’s Modified Eagle’s Medium) with added glycerol (0–8 M). The tissue system is chosen as 3 mm thick porcine liver slices as well as 2 cm diameter cores which were either imaged fresh (3–4 h cold ischemia) or after loading with DMEM based glycerol solutions (0–8 M) for times ranging from hours to 7 days at 4°C. The X-ray attenuation is reported in Hounsfield units (HU), a clinical measurement which normalizes X-ray attenuation values by the difference between those of water and air. NaCl solutions from 0 to 23.3% wt/wt (i.e. water to eutectic concentration) were found to linearly correspond to HU in a range from 0 to 155. At −196°C the variation was from −80 to 95 HU while at −78.5°C all readings were roughly 10 HU lower. At 20°C NaCl and DMEM solutions with 0–8 M glycerol loading show a linear variation from 0 to 145 HU. After freezing to −78.5°C the variation of the NaCl and DMEM solutions is more than twice as large between −90 and +190 HU and was distinctly non-linear above 6 M. After freezing to −196°C the variation of the NaCl and DMEM solutions increased even further to −80 to +225 HU and was distinctly non-linear above 4 M, which after modeling the phase change and crystallization process is shown to correlate with an amorphous phase. In all tissue systems the HU readings were similar to solutions but higher by roughly 30 HU, as well as showing some deviations at 0 M after storage, probably due to tissue swelling. The standard deviations in all measurements were roughly 5 HU or below in all samples. In addition, two practical examples for CT use were demonstrated including: (1) glycerol loading and freezing of tissue cores and, (2) a mock cryosurgical procedure. In the loading experiment CT was able to measure the permeation of the glycerol into the sample at 20°C, as well as the evolution of distinct amorphous vs. crystalline phases after freezing to −196°C. In the mock cryosurgery example, the iceball edge was clearly visualized, and attempts to determine the temperature within the iceball are discussed. An added benefit of this work is that the density of these frozen samples, an essential property in measurement and modeling of thermal processes, was obtained in comparison to ice.









Similar content being viewed by others
References
Bhowmick S., C. A. Khamis, J. C. Bischof (1998). Response of a liver tissue slab to a hyperosmotic sucrose boundary condition: microscale cellular and vascular level effects. Ann. N Y Acad. Sci. 858: 147–162
Bidault N. P., B. E. Hammer, A. Hubel (2000). Rapid MR imaging of cryoprotectant permeation in an engineered dermal replacement. Cryobiology 40(1): 13–26
Boutron P. (1986) Comparison with the theory of the kinetics and extent of ice crystallization and of the glass-forming tendency in aqueous cryoprotective solutions. Cryobiology 23: 88–102
Choi, J. H., and J. C. Bischof. A quantitative analysis on the thermal properties of phosphate buffered saline with glycerol at subzero temperatures. Int. J. Heat Transfer In Review
Coger, R., and M. Toner. Preservation Techniques for Biomaterials. Handbook of Biomedical Engineering. Bronzoni, CRC Press. 1:1567–1577, 1995
Daniel B. L., K. Butts, and W. F. Block (1999). Magnetic resonance imaging of frozen tissues: temperature-dependent MR signal characteristics and relevance for MR monitoring of cryosurgery. Magn. Reson. Med. 41(3): 627–630
Fuller B. J., A. L. Busza, and E. Proctor. (1989). Studies on cryoprotectant equilibration in the intact rat liver using nuclear magnetic resonance spectroscopy: a noninvasive method to assess distribution of dimethyl sulfoxide in tissues. Cryobiology 26(2): 112–118
Gage A. A. (1992). Cryosurgery in the treatment of cancer. Surg. Gynecol. Obstet. 174(1): 73–92
Gilbert J. C., G. M. Onik, W. K. Hoddick, B. Rubinsky (1985). Real time ultrasonic monitoring of hepatic cryosurgery. Cryobiology 22(4): 319–330
Han B., A. Iftekhar, J.C. Bischof (2004). Improved cryosurgery by use of thermophysical and inflammatory adjuvants. Technol. Cancer Res. Treat. 3(2): 103–111
Han, B., D. Swanlund, and J. Bischof. Enhancement of Direct Cell Injury During Freezing AT-1 Tumor Tissues By Use of Eutectic Crystallization. Summer Bioengineering Conference, Miamia, Florida, 2003
Hey J. M., D. R. MacFarlane (1998). Crystallization of ice in aqueous solutions of glycerol and dimethyl sulfoxide 2: ice crystal growth kinetics. Cryobiology 37(2): 119–130
Hindmarsh J. P., Buckley C., Russell A. B., Chen X. D., Gladden L. F., Wilson D. I., Johns M. L. (2004). Imaging droplet freezing using MRI Chem. Eng. Sci. 59 (2004): 2113–2122
Hobbs P. V. (1974). Ice Physics. Clarendon Press, Oxford
Hubbell J. H. (1982) Photon Mass Attenuation and Energy-absorption Coefficients from 1 keV to 20 MeV Int. J. Appl. Radiot. Isot. 33: 1269–1290
ICRU. Tissue Substitutes in Radiation Dosimetry and Measurement. Report 44 of the International Commission on Radiation Units and Measurements (ICRU). Bethesda, MD, 1989
Isbell S. A., C. A. Fyfe, R. L. Ammons, B. Pearson (1997). Measurement of cryoprotective solvent penetration into intact organ tissues using high-field NMR microimaging. Cryobiology 35(2): 165–172
Karlsson J. O. M, E. Cravalho, M. Toner (1994). A model of diffusion-limited ice growth inside biological cells during freezing. J. Appl. Phys. 75: 4442–4455
Karlsson, J., and M. Toner. Cryopreservation. Principles of Tissue Engineering. Vacanti and Langer. San Diego, Academic Press, 2000, pp. 293–307
Lonsdale K. (1958). The structure of ice Proc. R. Soc. A247: 424–434
Loser T., R. Wajman, D. Mewes (2001). Electrical capacitance tomography: image reconstruction along electrical field lines. Meas. Sci. Technol. 12: 1083–1091
Morneburg, H., Ed. Bildgebende Systeme für die medizinische Diagnostik. München, Publicis MCD Verlag, 1995
Mucal on the web http://www.csrri.iit.edu/mucal.html
Nerem R. (2000). Tissue Engineering: confronting the transplantation crisis. Proc. Instn. Mech. Engrs 214: 95–99
NIST data base of x-ray attenuation coefficitents. http://physics.nist.gov/PhysRefData/XrayMassCoef/cover.html
Onik G. (2001). Image-guided prostate cryosurgery: state of the art. Cancer Control 8(6): 522–31
Onik G., J. Gilbert, W. Hoddick, R. Filly, P. Callen, et al. (1985). Sonographic monitoring of hepatic cryosurgery in an experimental animal model. Am J Roentgenol 144(5): 1043–1047
Onik G., B. Rubinsky, R. Zemel, L. Weaver, D. Diamond, et al. (1991). Ultrasound-guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Preliminary results. Cancer 67(4): 901–907
Otten D.M., B. Rubinsky (2000). Cryosurgical monitoring using bioimpedance measurements–a feasibility study for electrical impedance tomography. IEEE Trans. Biomed. Eng. 47(10): 1376–1381
Ozisik M.N. (1994). Finite Difference Methods in Heat Transfer. Boca Raton, CRC Press
Pease G.R., S.T. Wong, M.S. Roos, B. Rubinsky (1995). MR image-guided control of cryosurgery. J. Magn. Reson. Imaging 5(6): 753–760
Petritsch, G., N. Reinecke, and D. Mewes. Visualization Techniques in Process Engineering. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Wiley VCH Verlag, 1999
Ravikumar T. S., R. Kane, B. Cady, R. L. Jenkins, W. McDermott, et al. (1987). Hepatic cryosurgery with intraoperative ultrasound monitoring for metastatic colon carcinoma. Arch Surg 122(4): 403–409
Sandison G. A., M. P. Loye, J. C. Rewcastle, L. J. Hahn, J. C. Saliken, et al. (1998). X-ray CT monitoring of iceball growth and thermal distribution during cryosurgery. Phys Med Biol 43(11): 3309–3324
Seibert, J. A., and J. M. Boone. X-Ray Imaging Physics for Nuclear Medicine Technologists. Part 2: X-Ray Interactions and Image Formation. J. Nucl. Med. Technol. 33:3–18, 2005
Tacke J., R. Speetzen, I. Heschel, D. W. Hunter, G. Rau, et al. (1999). Imaging of interstitial cryotherapy–an in vitro comparison of ultrasound, computed tomography, and magnetic resonance imaging. Cryobiology 38(3): 250–259
Walcerz D. B., M. J. Taylor, A. L. Busza (1995). Determination of the kinetics of permeation of dimethyl sulfoxide in isolated corneas. Cell Biophys 26(2): 79–102
Wang G., M. Vannier (2001) Micro-CT scanners for biomedical applications: an overview. Adv. Imaging 16: 18–27
Whitehouse R. W., G. Economou, J. E. Adams (1993). Influence of Temperature on QCT: Implications for Mineral Densitometry. J. Computer Assist. Tomogr. 17(6): 945–951
Acknowledgments
Thanks to Dr. Alptekin Aksan for a careful read of the manuscript. Funding is gratefully acknowledged from the Alexander von Humboldt Fellowship to JCB. Thanks to the Institute of Process Engineering (IfV) at the University of Hannover for hosting JCB during his Humboldt Fellowship in Spring 2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bischof, J., Mahr, B., Choi, J. et al. Use of X-ray Tomography to Map Crystalline and Amorphous Phases in Frozen Biomaterials. Ann Biomed Eng 35, 292–304 (2007). https://doi.org/10.1007/s10439-006-9176-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9176-7