Abstract
Oncoids from two localities (Ogrodzieniec and Blanowice) of the Polish Jura, southern Poland, have been investigated with respect to their genesis and paleoecology. These oncoids occur within Middle Jurassic (Bathonian) deposits. Those from Ogrodzieniec are large, elliptical, and embedded within a presumably condensed carbonate bed. Those from Blanowice, on the contrary, are significantly smaller, irregular to box-like in shape, and occur within the ore-bearing clays. The oncoids from both localities consist of a distinct carbonate core and laminated cortex that is significantly thicker and better preserved in the Ogrodzieniec oncoids. SEM and optical microscopic investigation of the oncoid cortices revealed the presence of carbonate and silicate layers with web-like structures similar to those occurring in recent cyanobacterial microbialites. Thus, the oncoid cortices investigated may have formed in a photic zone environment with the aid of coccoid and filamentous cyanobacteria. Oxic conditions prevailed during oncoid cortex formation within the siliciclastic setting, which is manifested by low total organic carbon content, high pristane/phytane (Pr/Ph) ratio, and significant predomination of the C31 homohopanes. On the cortices’ surfaces, as well as between particular laminae, various encrusting organisms have been found. The encrusters, dominated by serpulids and bryozoans, are cryptic species that inhabited the undersides and recesses of the oncoids. Their presence on both the upper and lower surfaces of the oncoids indicates that the oncoids were episodically overturned on the seafloor. The much better developed cortex lamination and much higher diversity and abundance of encrusters in the Ogrodzieniec oncoids may point to better trophic conditions prevailing in a shallower marine environment characterized by transparent waters, as opposed to a deeper siliciclastic environment with less transparent waters and probably worse trophic conditions prevailing during formation of the Blanowice oncoids.
Similar content being viewed by others
Introduction
Oncoids (or oncoliths, e.g., Palmer and Wilson 1990) are biosedimentary structures in a form of generally spherical nodules, usually composed of a laminated micritic cortex enveloping a biogenic or abiogenic nucleus (core) (e.g., Flügel 2004). According to the definition given by Peryt (1983) and Tucker and Wright (1990), oncoids are irregularly shaped coated grains with a diameter greater than 2 mm. They may have formed in both marine and freshwater environments (e.g., Brand and Veizer 1983; Nickel 1983). Usually, oncoids are reported from shallow-water (under photic and wave agitation conditions) environments being formed by photosynthetic cyanobacteria and algae (e.g., Peryt 1981, 1983; Riding 1983; Tucker and Wright 1990; Védrine et al. 2007; Schlagintweit and Gawlick 2009; Bádenas and Aurell 2010; Olivier et al. 2011). Riding (1983, see also Herbig 1994) even called such oncoids ‘cyanoids’, a term that provided a more genetic sound to the general term ‘oncoids’. However, oncoids are also known from deeper-water settings and they are interpreted to have been formed by non-photosynthetic microorganisms (like bacteria and fungi) in dim or completely dark environments (e.g., Dromart et al. 1994; Gradziński et al. 2004; Reolid et al. 2005; Reolid and Nieto 2010; Reolid et al. 2010). It must be noted as well that such ‘microbial coated grains’ may also be co-formed by benthic foraminifers (e.g., Gradziński et al. 2004; Reolid et al. 2005).
Recently, medium- to large-sized oncoids intensively encrusted by diverse sessile invertebrates (sclerobionts sensu Taylor and Wilson 2002, 2003) have been found in the Middle Jurassic (Bathonian) deposits of the Polish Jura in southern Poland. Using field observations and microscopic study of the oncoid cores and cortices, we provide data about their formation in both carbonate and siliciclastic settings. A geochemical study conducted on one of the studied sections provided detailed data about the siliciclastic environment in which some of the oncoids formed. Qualitative and semi-quantitative paleoecological analyses of the oncoid-hosted encrusting biota then provide new information concerning the diversity and abundance of benthic sessile organisms, as well as their relationship to the oncoid substrate.
Geological setting
Geological background
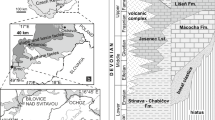
The Polish Jura area forms a belt of Jurassic deposits extending from the southeast to northwestern direction in southern and south-central Poland (Fig. 1a). The epicontinental Upper Bajocian through Bathonian deposits occur as a monotonous sequence of unconsolidated dark-grey to black clays, intercalated with numerous, more or less continuous horizons of carbonate concretions and massive siderite beds (e.g., Majewski 2000; Matyja and Wierzbowski 2000; Zatoń et al. 2009). These deposits are referred to as the Ore-bearing Częstochowa Clay Formation (see e.g., Dayczak-Calikowska et al. 1997; Kopik 1998; Matyja and Wierzbowski 2000). Currently, they are exposed in numerous clay pits, the majority of which are still active, scattered across the Polish Jura.
a Map of Poland with Jurassic deposits indicated (shaded areas) after removal of the Cenozoic cover. b Simplified geological map of a part of the Polish Jura showing location of the areas investigated (stars); 1 Triassic, 2 Lower Jurassic, 3 Middle Jurassic, 4 Upper Jurassic, 5 Cretaceous. c Simplified locality sketch-map of the Ogrodzieniec-Świertowiec outcrops investigated. d Simplified locality sketch-map of the Blanowice clay pit investigated
The oncoids investigated here have been detected in two localities of the Polish Jura: (1) in a working clay pit at Blanowice near Zawiercie (Fig. 1b, d), (2) in small outcrops recently excavated by ‘fossil hunters’ in Ogrodzieniec-Świertowiec (simply called Ogrodzieniec) (Fig. 1b, c). Both localities are situated in the southern part of the Polish Jura area and are confined to the southern (Ogrodzieniec) and the southern part of the northern (Blanowice) sedimentary regions (sensu Różycki 1953). The southern sedimentary region is characterized by greater facies variability, thinner deposits, and common hiatuses. The area of Ogrodzieniec is confined to the more near-shore facies zone of the ore-bearing clays. The northern sedimentary region, on the other hand, is characterized by a more fully developed and thicker sequence of the ore-bearing clays (see Różycki 1953). The clays in the northern sedimentary region are rather monotonous with respect to facies, with no obvious variation in the field. The depositional environment of the clays has been interpreted as quiet marine, generally below the storm wave-base (Matyja et al. 2006a, b, c), although storm episodes are evidenced in several horizons of the ore-bearing clays in the form of bored and encrusted concretions bearing signs of intense abrasion (see Zatoń et al. 2006a, 2011).
Characteristics of the sections studied
Blanowice section
The oncoids (bed 6, see Fig. 2) from Blanowice occur in dark-grey clays with a sandy fraction, just above or in transition with poorly lithified, clayey sandstones (bed 5). The oncoids form one nearly continuous horizon. The oncoids lay close to each other, generally with their long axis parallel to the bedding. Above, a horizon of siderite concretions with ooids occurs (bed 8, see Fig. 2). Just below the oncoids, the ammonite Asphinctites, characteristic for the uppermost Lower Bathonian (Tenuiplicatus Zone), was detected. Above the oncoid horizon, on the other hand, the ammonite Tulites cadus, characteristic for the Middle Bathonian (Subcontractus Zone), was also found (Zatoń 2007). This implies that the oncoids investigated are confined to the interval between the Lower and Middle Bathonian (Figs. 2, 3a). Zaton (2007, fig. 2) tentatively included the horizon in the uppermost Lower Bathonian. However, due to the common hiatuses and condensation of the sediments in the area under discussion (see Różycki 1953), the precise stratigraphic position of the oncoid horizon is uncertain.
a The sampled section at Blanowice with ammonite zones delineated and oncoid horizon indicated (arrow). Hammer at the center is 30 cm long. b Basal carbonate bed (bed 1 on Fig. 2) from Ogrodzieniec with in situ preserved stromatolite-like structure, large oncoids (indicated with dashed lines), small oncoids and limonitized concretions above (arrowed). c Example of an oncoid from Blanowice with partially worn out cortex. d Large, discoidal oncoid from Ogrodzieniec heavily encrusted mainly by serpulid/sabellid worm tubes. e Cross section of the Blanowice oncoid showing large core and thin cortex, separated from each other by intermittent lines. f Cross section of the discoidal Ogrodzieniec oncoid showing its flat-shaped core and thick cortex, separated from each other by intermittent line
Ogrodzieniec section
The oncoids from Ogrodzieniec mainly occur within a thick (up to 20 cm in thickness) sandy limestone bed that is the most basal part of the section visible (bed 1, Fig. 2). Within the bed, large oncoids are observed to form one distinct horizon, although some small (a few cm in diameter) ones were also found scattered above the large ones. The latter were observed to lie horizontally, oblique, and even vertically with respect to bedding (Fig. 3b). Locally, in the lowermost part of bed 1, just below the oncoid horizon, a thick laminated stromatolite-like crust can occur (Fig. 3b) that originally encrusts the massive limestone bodies, some of which possess Gastrochaenolites borings. Just above the oncoid horizon, irregular grey carbonate clasts with limonitic encrustation may occur (Fig. 3b). Throughout the bed, small carbonate clasts with distinct boring traces, small (millimeter to half of centimeters in size) quartz pebbles and variously preserved (including fragmented) fossils (bivalves, brachiopods, ammonites, belemnites, many of which are encrusted) some of which were reworked, also occur. The bed can be traced, albeit irregularly due to the limited number of small exposures, for a distance of ca. 100 m. The top of bed 1 is characterized by an uneven surface with many shallow depressions, coated with limonitic encrustation. The features of the limestone bed provided above are characteristic of condensed deposits (e.g., Szulczewski 1968; Gatrall et al. 1972; Giżejewska and Wieczorek 1976; Aurell et al. 1994; Reolid et al. 2010).
The limestone bed is overlain by a thin clay where one oncoid has been found (bed 2), but due to the poor state of the exposure, there is no certainty whether oncoids really occur above this bed. The clays are overlain by thin, rusty and irregular siderite concretions (bed 3), above which the clay sequence is visible (bed 4, Fig. 2). However, due to poor exposure of this part of the section (vegetation and slump cover), no details about its lithological variation can be deduced.
In the basal limestone bed, fragments of the ammonite genera Parkinsonia (Parkinsonia) and P. (Durotrigensia) were found. Additionally, one fragment of Parkinsonia (Oraniceras) gyrumbilica was also found ex situ but certainly derived from the bed. That means the oncoids may be confined to the Lower Bathonian and may not be younger than the Macrescens Subchron of the Zigzag Chron.
Materials and methods
Sampling and analytical methods
The oncoids were collected from the section exposed at Blanowice during spring 2007. As the exposure is characterized by its very limited lateral extent (ca. 2 m), only 30 specimens were collected for paleoecological investigation. Due to the nature of the host clay sediment (unconsolidated and wet), the oncoids collected are poorly preserved with respect to their cortex—in many places it has been detached from them in the section and/or it has been eroded away.
Because the oncoids from Ogrodzieniec occur within a hard carbonate bed, first the fragments of the bed were excavated and then the oncoids were collected (summer 2009). Many times the oncoids were retrieved from the excavated material after several weeks because the weathering and washing by rain enabled easier collection. The preservation of the oncoids from Ogrodzieniec is much better than those from Blanowice. Their outer part (cortex) is complete in nearly all specimens collected. In total, 40 large, well-preserved oncoids were collected.
The oncoids were examined in standard petrographic thin sections using optical and scanning electron microscopy (SEM) at 20 kV voltage. For SEM observations, a series of rock platelets was etched with 8% formic acid for 30 s and 40% hydrofluoric acid for 60 s. The SEM examinations were performed using a Philips XL 20 scanning microscope equipped with an ECON 6 EDX-DX4i detector and a Zeiss DSM 960A equipped with Bruker axs EDS detector. The mineralogical composition of the oncoids was analyzed using the Philips PW 3710 diffractometer. Selected encrusters were imaged in uncoated state using Philips XL 30 environmental scanning electron microscope (ESEM) in a back-scattered electron (BSE) mode, and in a coated state (ammonium chloride) using a Canon digital camera.
In order to carry out the paleoecological study, all the oncoids collected were inspected under a binocular microscope. All encrusting organisms have been identified to the lowest taxonomic level possible, and all encrusters were counted and their relative frequency was presented following the procedure of Schlögl et al. (2008): <10 specimens as rare, 10–30 as present, 31–100 as common and >100 specimens as abundant occurrences. The relative frequency of encrusters was separately given for all 30 examined oncoids from Blanowice and 40 oncoids from Ogrodzieniec. This abundance data follows the methods of Palmer and Wilson (1990).
Organic geochemistry
For total organic carbon (TOC) and biomarkers, only the section at Blanowice was sampled due to its much better exposure and prevalence of suitable oncoid-hosted sediments (clays). The oncoid cortices from Ogrodzieniec (three samples) and Blanowice (one sample) were also analyzed in order to determine bulk geochemical data and detect some specific biomarkers (Table 1).
TOC
Total Organic Content (TOC) was determined using an Eltra Elemental Analyser model CS530. TOC was calculated as total carbon (TC) minus total inorganic carbon (TIC). TC and TIC were measured using an infrared cell detector of CO2 gas, which was evolved by combustion under an oxygen atmosphere for TC, and was obtained from reaction with 50% phosphoric acid for TIC. Calibration was made by means of Eltra standards. Calcium carbonate content was calculated as CaCO3 = 8.333 × TIC, assuming that all carbonate is present as calcite. In total, nine samples derived from oncoid cortices, carbonate nodules and host sediment were analyzed (Table 1).
Extraction and separation
The clays were Soxhlet-extracted in pre-extracted cellulose thimbles with dichloromethane. Extracts were further separated using preparative pre-washed TLC plates coated with silica gel (Merck, 20 × 20 × 0.25 cm and 10 × 20 × 0.25 cm). Prior to separation, the TLC plates were activated at 120°C for 1 h. The plates were loaded with the n-hexane soluble fraction and developed with n-hexane. Bands comprising aliphatic (Rf 0.4–1.0), aromatic (Rf 0.05–0.4) and polar (Rf 0.0–0.05) fractions were collected.
Gas chromatography—mass spectrometry (GC–MS)
The GC–MS analyses were performed with an Agilent 6890 Series Gas Chromatograph interfaced to an Agilent 5973 Network Mass Selective Detector and Agilent 7683 Series Injector (Agilent Technologies, Palo Alto, CA). A 0.5 μl sample was introduced into the cool on-column injector under electronic pressure control. Helium (6.0 Grade, Linde, Kraków) was used as the carrier gas at a constant flow rate of 2.6 ml/min. The GC separation was on either of two fused-silica capillary columns:
(1). J&W HP5-MS (60 m × 0.32 mm i.d., 0.25 μm film thickness) coated with a chemically bonded phase (95% polydimethylsiloxane, 5% diphenylsiloxane). The GC oven temperature was programmed from 40°C (isothermal for 1 min) to 120°C at a rate of 20°C/min, then to 300°C at a rate of 3°C/min. The final temperature was held for 35 min.
(2). J&W DB17-MS (60 m × 0.25 mm i.d., 0.25 μm film thickness) coated with a chemically bonded phase (50% phenyl-methylpolysiloxane). The GC oven temperature was programmed from 50°C (isothermal for 1 min) to 120°C at a rate of 20°C/min, then to 300°C at a rate of 3°C/min. The final temperature was held for 45 min.
The GC column outlet was connected directly to the ion source of a mass spectrometer. The GC–MS interface was kept at 280°C, while the ion source and the quadrupole analyzer were at 230 and 150°C, respectively. Mass spectra were recorded at 45–550 da (0–40 min) and 50–700 da (above 40 min). The mass spectrometer was operated in the electron impact mode (ionization energy: 70 eV).
Quantification and identification
An Agilent Technologies Enhanced ChemStation (G1701CA ver.C.00.00) and the Wiley Registry of Mass Spectral Data (7th Edition) software were used for data collection and mass spectra processing. The abundances of the selected compounds were calculated by comparisons of peak areas for internal standard (9-phenylindene, Aldrich) with the peak areas of the individual hydrocarbons obtained from the GC–MS ion chromatograms. Identification of individual compounds was aided by comparison with published mass spectra and by interpretation of mass spectrum fragmentation patterns.
Results
Morphology, size and composition of the oncoids studied
Oncoids from Blanowice
The oncoids are generally pebble to cobble-sized and of various shapes. They are small (ca. 4 cm long) and oval to nearly equilateral, or larger (ca. 11 cm long and 8 cm wide), of box-like to irregular appearance (Fig. 3c, e), and may be termed macrooncoids (or macroids, see Flügel 2004). The oncoids are composed of two main components: a core and cortex (envelope). The core consists of micritic carbonate matrix in which bioclasts, such as numerous echinoderm ossicles, foraminifers and shell fragments, as well as lithoclasts, such as quartz grains and ooids occur. The ooids are up to 1 mm in diameter, consisting of tiny core and well-developed concentric calcitic laminations. The core may be composed of casts of infaunal bivalves as well. Some cores possess Gastrochaenolites bivalve borings. Mineralogically, the core of the oncoids consists primarily of high Mg-calcite (~10% mol MgCO3). The rest of the mineral phases present consist of gypsum, kaolinite, muscovite or illite, pyrite and quartz.
The cortex, up to a few mm thick, is composed of planar to wavy laminae. In transmitted light, the lamination primarily consists of alternations of reddish-brown and light-grey laminae. The laminae may be folded and discontinuous. Often, the wavy reddish-brown laminae form domal structures that are visible in thin sections and on the oncoid surface. Often the laminae alternate with encrusting organisms, of which the serpulid tubes or foraminifer tests are easily seen in thin sections (Fig. 4a). The cortex consists primarily of high Mg-calcite, as evidenced from both the XRD and EDS analyses. Quite common are also crandallite (phosphate) and sphalerite. Commonly, the cortex of the Blanowice oncoids is worn out in many places (Fig. 3c, e).
a Transmitted light microscope image of an oncoid from Blanowice showing wavy lamination and encrusting biota preserved between the laminae in the cortex (arrowed), and numerous bio- and lithoclasts in the core. b SEM photomicrograph of laminated cortex of the oncoid from Ogrodzieniec with well-visible spaces after the skeletons of encrusting biota (arrowed) that were dissolved using a weak HCl during etching
Morphologically, the oncoids from Blanowice are similar to the ‘snuff-boxes’ described by Gatrall et al. (1972) and Palmer and Wilson (1990), and to the oncoidal ferruginous microbialites described by Burkhalter (1995).
Oncoids from Ogrodzieniec
Unlike the oncoids from Blanowice, those from Ogrodzieniec are much larger (the largest measures 25 × 15 cm) and thinner (generally 4.5–6.5 cm thick), and their shape may range from discoidal to ellipsoidal (Fig. 3d, f), rarely spherical. The limestone inner core is full of bioclasts, lithoclasts and ooids as in the case of the Blanowice oncoid cores. In one case the core consisted of an ammonite fragment.
The cortex is formed by planar to wavy laminae, from a few mm to 2 cm in thickness and in most cases asymmetrical. Single cores were bored by bivalves as well. In transmitted light, the lamination is very similar to that in the Blanowice oncoids, consisting of alternations of reddish-brown and lighter-colored laminae. The individual layers or packets of layers can be folded, wrapped and discontinuous. They are also distinctly thicker on only one side of the oncoid, or in its lateral margins. As in the Blanowice oncoids, the laminae alternate with encrusting organisms (Fig. 4b). Mineralogically, the crust of the Ogrodzieniec oncoids is similar to that from Blanowice. The main mineral of the crust is high Mg-calcite, associated with well-ordered kaolinite (~15–17%). It is also confirmed by the EDS analyses of particular laminae, some of which are composed primarily of CaCO3 and some by higher amount of aluminosilicates. In trace amounts, phosphate crandallite (~2–3%), illite (~1%) and quartz (up to 1%) are present, as well.
Morphologically the oncoids are quite similar to some of the larger ‘snuff-boxes’ described by Gatrall et al. (1972) and the ferruginous oncoids of Gradziński et al. (2004).
Detailed characteristics of the oncoid cortices
Two alternating types of laminae—carbonate and silicate—can be distinguished in the oncoid cortices from Blanowice and Ogrodzieniec when investigated under the SEM (Fig. 5b, c). The thickness of layers, which varies from ca. 1 to 10 μm and more, does not seem to depend on mineral composition, although commonly the carbonate layers are thinner than the silicate ones (Fig. 5a, b).
Oncoids from Ogrodzieniec and Blanowice. a Transmitted light image of oncoid envelope, Ogrodzieniec. b–g SEM images presenting detail morphology of oncoid envelopes. b HF-etched sample showing regular lamination of carbonate (thin) and silicate (thicker) layers. EDAX spectra show differences in composition between both types of layers, Ogrodzieniec. c Fragment of silicate layer (magnified in d) forming web-like structure with silicate granules within it, Ogrodzieniec. d Magnified fragment of silicate layer, the web-like structure is indicated by long arrows, whereas silicate granules indicated by small arrows, Ogrodzieniec. e Fragment of carbonate layer (etched with hydrofluoric acid) revealing web-like structures (arrowed) where walls are permineralized by silicates, whereas carbonates infilling pits are dissolved by formic acid etching, Blanowice. f Granules forming the silicate layers, Ogrodzieniec. g Remnants of filamentous organic structures identified within silicate and carbonate layers, Ogrodzieniec
The carbonate layers are composed mainly of small, 3–6 μm, calcite crystals with larger, block-shaped calcite crystals forming thicker layers. SEM examination of polished platelets etched with weak formic acid revealed a distinct irregular web-like pattern in some thicker carbonate layers (Fig. 5e).
The silicate layers (Fig. 5b–d) are composed of sheeted phyllosilicates and granular silicate minerals. EDAX analyses revealed Si, Al, K, Ca and Mg in the minerals forming these layers. Due to the low concentration of calcium carbonate in the silicate layers, etching with formic acid produced no relief. Etching with hydrofluoric acid showed clear morphological diversification of the silicate layers and revealed an irregular web-like pattern, with walls of various thicknesses, from 2 to 6 μm, and pits with diameters from 4 to 10 μm. Walls around the pits are composed of platy phyllosilicates whose size varies from 6 to 40 μm. The other components of the silicate layer are minute granules with compositions similar to those of the phyllosilicates (Fig. 5d, f). The size of these granules is relatively constant, averaging about 2–5 μm. The granules are distributed densely within almost all silicate layers and occur also within the walls of the web-like structures (Fig. 5d). They are also found sporadically in carbonate layers.
In both carbonate and silicate layers, carbonaceous organic remnants are occasionally preserved. They are represented by short filaments, 10 μm diameter, and patches of web-like irregular structures (Fig. 5g). However, their preservation is poor and insufficient for precise morphological identification.
Organic geochemistry
The organic geochemistry was performed using the clay samples derived from the oncoid- and carbonate concretion-bearing horizons, as well as from intercalating horizons at the Blanowice clay pit (Fig. 2). Three oncoid cortices from the Ogrodzieniec outcrop were analyzed for TOC and carbonate content. Bulk geochemical data is shown in Table 1. Significant differences in the samples analyzed from the Blanowice section are reflected in the CaCO3 content and TOC concentration. The oncoid- and concretion-bearing horizons are enriched in CaCO3, the concentration of which ranges from 21 to 26% (Table 1). The samples coming from intercalating horizons contain ca. 5.5% CaCO3, the concentration of which is very similar in all of the three samples analyzed (Table 1). There is a large concordance of TOC concentration for the clay samples coming from the concretion-bearing and intercalating horizons. The samples that are enriched in CaCO3 contain 2.2–2.3% TOC, and the clays that intercalate them contain 2.6–2.8% TOC (Table 1; Fig. 2). On the other hand, the oncoids from the Ogrodzieniec outcrop contain very low TOC concentrations, not exceeding 0.1% (Table 1) and much higher carbonate content (up to 79%). Very low TOC concentration and macroscopic observations suggest weathering processes influenced the oncoid cortices from Ogrodzieniec.
The other elementary geochemical features, such as extractable organic matter (EOM) content, group composition of extracts and basic molecular parameters based on distribution of n-alkanes and isoprenoids like carbon preference index (CPI), pristane to phytane ratio and pristane to n-heptadecane and phytane to n-octadecane ratio (Table 1) are very similar for the all samples analyzed. Two characteristic carbon number maxima are observed in n-alkanes distributions. The first is between n-C16-n-C19 and the second, from n-C25-n-C29. In the case of the samples analyzed, n-alkanes from the n-C25-n-C29 range predominated over that of low molecular weight (Fig. 6). The carbon preference index (CPI and CPI(25–31)) values are significantly higher than 1 for all samples and in the case of CPI(25–31) parameter are even >2 (Table 1). The distribution of isoprenoids (pristane—Pr and phytane—Ph) in relation to n-alkanes is characterized by low to medium values of Pr/n-C17 and Ph/n-C18 (Table 1), reaching slightly above or below 1, respectively.
Partial mass chromatograms of a m/z 71 showing the distribution of n-alkanes in the oncoids horizon and overlying clay of the Blanowice section. Note the strong predomination of even versus odd high molecular weight n-alkanes and b Partial mass chromatograms of the m/z 191 showing hopanes and hopenes distribution in the investigated samples. Note the very similar distribution of these compounds in both samples. HP-5MS column was used
As is typical for the clays analyzed here, the most abundant hopanes are 17β,21β-hopane and norneohop-13(18)-ene (Fig. 6, Marynowski et al. 2007). The distribution of C31 to C35 homohopanes is characterized by a significant predomination of the C31(22S+22R) homologues and strong excess of the less stable R epimer (Fig. 6). Moreover, hopanes with 35 carbon atoms in the molecule are not detected in any sample, and hopanes with 34 carbon atoms are only present in trace amounts.
In the all clay samples analyzed, fernenes, the natural product biomarkers first identified in ferns, were identified (Ageta and Arai 1983; Paull et al. 1998; Marynowski et al. 2007). Among the steroids, the diaster-13(17)-enes (20R and 20S epimers) and 4β-methyldiaster-13(17)-enes (20R and 20S epimers) are clearly dominant in all clays, while ∆4 and ∆5 sterenes, steranes and diasteranes are not found.
Perylene is definitely the dominant compound among the polycyclic aromatic hydrocarbons (PAHs) in these samples (Table 2). Methyl- (m/z 266) and dimethyl- (m/z 280) derivatives of perylene are also present in relatively high concentrations, probably representing diagenetic methylation products (Alexander et al. 1995). Other quantitatively important aromatic biomarkers are cadalene, dehydroabietane, simonellite and retene, and also other sesquiterpenoids such as calamenene and cadina-1(10),6,8-triene that were found only in trace amount. In addition, other common aromatic constituents of the clay sedimentary organic OM include the PAHs: phenanthrene, fluoranthene, pyrene and their alkyl derivatives as well as the more condensed PAHs: benzo[g,h,i]fluoranthene, benz[a]anthracene, chrysene + triphenylene, benzo[e]pyrene, benzo[a]pyrene, indeno(1,2,3-cd)pyrene and benzo[ghi]perylene. Concentrations of the quantitatively most important aromatic biomarkers and PAHs are shown in Table 2.
Encrusting organisms
The entire faunal spectrum inhabiting the oncoids consists of encrusting organisms (sclerobionts sensu Taylor and Wilson 2002) that also inhabited other firm and hard substrates during the Jurassic, such as hardgrounds, hiatus concretions, cobbles and skeletal remains. All the detected sclerobionts are listed in the Table 3. The sclerobionts encrusting the Bathonian oncoids from Blanowice and Ogrodzieniec consist of the following groups:
Foraminifera
They are represented by rather rare occurrences of representatives of the genus Vinelloidea (=Nubeculinella). These foraminifers consist of uniserial to multilocular, calcareous chambers, oval in shape. The low numbers of foraminifers encrusting the oncoid surfaces may be a result of their general rarity on such substrates in these environments because even on such well-preserved oncoids, as occur in Ogrodzieniec, they are only sporadic (Table 3; Fig. 7).
Sponges
A few (<10 specimens) unidentified cone-like calcisponges resembling ‘sclerosponges’ were detected encrusting the oncoids from Ogrodzieniec only (Fig. 8a).
Selected sclerobionts encrusting the Bathonian oncoids studied. a Tiny calcisponge, Ogrodzieniec. b A base of solitary ?caryophyllid scleractinian coral (arrowed), Ogrodzieniec. c Entangled tube of a sabellid polychaete Glomerula gordialis, Blanowice. d Serpulids Propomatoceros sp. associated with thecideide (?Rioultina sp.) brachiopods (arrowed), Ogrodzieniec. e Cyclostome bryozoan ‘Berenicea’ (white arrow) associated with serpulids Cementula sp. (black solid arrow) and sabellid Glomerula sp. (black intermittent arrow), Ogrodzieniec. f Enigmatic tubular fossils, Blanowice
Corals
On the oncoids the corals are represented by single specimens, preserved as attachment bases with easily visible septation (Fig. 8b). They are very similar to ?caryophyllid scleractinians reported from the Middle Jurassic hiatus concretions of the Polish Jura (Zatoń et al. 2011).
Sabellid and serpulid worm tubes
This is the most abundant group of encrusters on the oncoids (Table 3; Fig. 7). Eight morphological genera are recognized. There are probably more species but detailed taxonomic work is needed. The sabellid Glomerula gordialis is a common tube encrusting both the Blanowice and Ogrodzieniec oncoids. It is characterized by tubes with entangled patterns of encrustation (Fig. 8c), especially at the beginning of their development (see Radwańska 2004). Glomerula sp. consists of thin tubes, circular in cross section, forming gentle to curving trails on the oncoid surface. It is similar to Cycloserpula flaccida presented by Ippolitov (2007a). Generally, G. gordialis and G. sp. are the most abundant tubes on the Blanowice and Ogrodzieniec oncoids (Table 3). The serpulid Propomatoceros sp. (see e.g., Ippolitov 2007b) occurs as a tube bearing a distinct keel on its dorsal side (Fig. 8d), making the cross section triangular or with slightly rounded margins. Propomatoceros sp. may be common to abundant on particular oncoids from Ogrodzieniec. Rare on both Blanowice and Ogrodzieniec oncoids, Nogrobs sp. is characterized by tube of quadrate cross section.
Cementula sp. consists of tightly spirally coiled tubes (Fig. 8e) that differ from Spiraserpula in lacking internal ridges (see Pillai 1993). On particular oncoids from Ogrodzieniec this morphogenus may be common. Filogranula sp. is here represented by small pentagonal tubes possessing distinct dorsal and lateral keels, and with a petaloid aperture (see Ippolitov 2007a). This morphogenus is a rare faunal component and was detected encrusting only the Ogrodzieniec oncoids. The similar ?Metavermilia sp. consists of larger and long tubes possessing three or more keels. The characteristic petaloid aperture known in Filogranula has not been observed here. Rare ?Mucroserpula sp. consists of individuals with short, robust tubes, that curve in a form of an ellipse, possessing nearly triangular cross section with rounded lateral sides, with a dorsal keel (see Ippolitov 2007b for detailed description). Serpulids provisionally determined as ‘Serpula’ sp., are represented by thin tubes, circular or nearly circular in cross section, possessing collars. This species occur rarely on the Ogrodzieniec oncoids.
Many serpulid and sabellid tubes encrusting the oncoids from Blanowice are strongly abraded (only their bases are visible), so their generic or specific determination is not possible. All the worm tubes encrust the lower, upper and lateral oncoid surfaces.
Enigmatic tubes
These tubular fossils possess characteristic depressions on their surfaces in a honeycomb pattern (Fig. 8f). The fossils are very common on particular oncoids (Table 3; Fig. 7), and may differ in the tube diameter and length. The tubes are aligned close to each other and may encrust each other. Currently, it is not known to which group they belong. The honeycomb-like depressions on the tube surfaces are not known in any of the fossil and recent serpulids (Olev Vinn, pers. comm. 2010), so they are treated here as incertae sedis. More research is needed to determine their affinity. Similar tubes were found encrusting the microbial crust-bearing hiatus concretions (Zatoń et al. 2011).
Bivalves
Bivalve traces are relatively rare on the oncoids (Table 3). In the case of Blanowice, it may be because large portions of the oncoid crusts are not preserved, but this seems to be of secondary importance as cementing bivalves are rare even on the well-preserved surfaces of the oncoids from Ogrodzieniec. The traces of bivalves consist of fragmentary lower valves that resemble those of Plicatula and Liostrea (here assigned tentatively as ‘Liostrea’ Fig. 9a). They encrust the lateral sides and open upper and lower surfaces of the oncoids.
Bryozoans
They are represented by cyclostomes, the only skeleton-bearing bryozoan group present in the Jurassic (see Taylor and Ernst 2008). The cyclostomes encrusting the oncoids from Ogrodzieniec have been described recently by Zatoń and Taylor (2010). Here, the bryozoans are represented by three colony-forms: (1) sheet-like bereniciform colonies, (2) uniserial and oligoserial runners and (3) dome-shaped or ‘flabellotrypiform’ colonies (see e.g., Taylor and Ernst 2008 for terminology). The dominant colony-forms are sheet-like bereniciforms. They are represented by encrusting, unilamellar or multilamellar and multiserial colonies. The most common is ‘Berenicea’ spp. (Table 3; Figs. 7, 8e, 9d), a form-genus including all bereniciforms lacking gonozooids (polymorphic zooids brooding the larvae which are very important in cyclostome taxonomy at the genus level; see Taylor and Sequeiros 1982). However, rarely, but present on the Ogrodzieniec oncoids, are fertile bereniciform colonies belonging to such genera as Microeciella, Hyporosopora and Reptomultisparsa (Fig. 7). Therefore, it may be assumed that all of the colonies lacking gonozooids in fact represent these genera. Generally, following the worm tubes, the berenicean colonies are the second most common group of encrusters on the oncoids, as exemplified on the Ogrodzieniec samples (Fig. 7). Uniserial and oligoserial colonies are a much more rare component among the bryozoans on the oncoids, represented by the genera Stomatopora and ?Proboscinopora, respectively. Among Stomatopora, two species may be recognized: S. bajocensis with slender, thin autozooids, and larger Stomatopora sp. (Fig. 9b). They were detected only in the Ogrodzieniec oncoids. The dome-shaped and ‘flabellotrypiform colonies’ belong to free-walled cyclostomes Ceriocava sp. that expand upwardly rather than laterally. Generally, this suite of bryozoans also encrusts hiatus concretions in the Polish Jura area (Zatoń and Taylor 2009a).
Selected sclerobionts encrusting the Bathonian oncoids studied. a Oyster attachment valves heavily encrusted by different sclerobionts, Ogrodzieniec. b Cyclostome bryozoan Stomatopora sp. overgrown by the colony of ‘Berenicea’ in the lower right, Ogrodzieniec. c Tubes of the serpulid Propomatoceros sp. associated with numerous thecideide (?Rioultina sp.) brachiopods (arrowed), Ogrodzieniec. d Association of different groups of sclerobionts; bryo. bereniciform bryozoans, mic. microconchids Punctaconchus, serp. serpulid and sabellid worm tubes, thec. thecideide brachiopod (Thecidellinidae indet.), Ogrodzieniec
Microconchids
These Spirorbis-like tubes are very uncommon among the Jurassic encruster communities (see Vinn and Taylor 2007), and here as well they occur as single (three at most) individuals on particular oncoids from Ogrodzieniec only. In total, only 16 specimens have been found. Their tubes bear distinct punctae on the surface indicating the species Punctaconchus ampliporus Vinn and Taylor 2007 (see Zatoń and Taylor 2009b) (Fig. 9d). They distinctly encrust the oncoid crevices or lateral vertical margins, and never occur on the open upper or lower surfaces. Apart from the Ogrodzieniec oncoids, microconchids have not been detected in the Jurassic of Poland at all.
Brachiopods
Two species of thecideide brachiopods have been found on the oncoids from Ogrodzieniec. The first is represented by tiny, mostly juvenile individuals encrusting the crevices and depressions in the substrate. They are probably represented by Rioultina sp. (Figs. 8d, 9c). The second species is represented by larger individuals preserved mainly as their ventral valves only, and thus lacking the important diagnostic features. They are assigned as Thecidellinidae indet. (Fig. 9d). Generally, the thecideide brachiopods occur as single individuals preserved as either complete shells or cemented ventral valves on particular oncoids, but on one sample (Fig. 9c) their number exceeds 30. Taking the total number of encrusted oncoids, thecideides are the third most abundant group, following serpulids and bryozoans (Fig. 7). Thecideide brachiopods have not been noted from the Bathonian of Poland until this work.
Discussion
The origin of the Bathonian oncoids
Theories of microbial genesis of oncoids have been discussed in the literature many times. Oncoids are generally regarded as having formed through the activity of cyanobacteria (e.g., Peryt 1981; Riding 1983; Flügel 2004). The regular and distinct lamination of oncoid cortices resembles stromatolitic structures and points to a microbial role in their formation. Some oncoids contain fossilized remnants of cyanobacteria and algae, and it is commonly assumed that most calcareous oncoids were produced by these microorganisms in shallow-water environments. However, there are examples of oncoids forming in both shallow- (e.g., Gatrall et al. 1972; Palmer and Wilson 1990; Burkhalter 1995) and deep-water settings (e.g., Gradziński et al. 2004; Reolid and Nieto 2010; Reolid et al. 2010), the cortices of which are composed of iron oxide phases that are interpreted to have been precipitated by non-photosynthetic microbiota, like bacteria and fungi.
The both XRD and EDAX analyses of the cortices of the Bathonian oncoids from Blanowice and Ogrodzieniec have not revealed the presence of any iron oxides that could have been precipitated by bacteria or fungi (e.g., Palmer and Wilson 1990; Gradziński et al. 2004; Reolid and Nieto 2010). Instead, EDAX analyses revealed two alternating types of laminae—carbonate and silicate. Detailed SEM and optical microscope analyses confirm that web-like structures in oncoid cortices (present in both silicate and carbonate layers) represent mineralized exopolymeric substances (EPS) of cyanobacterial mats. The generation of individual laminae in the studied oncoids was most likely closely related to life activity of the microbial assemblage and may reflect some periodical (seasonal) changes in environment. The mineralogical differentiation correlates with changes of geochemical condition (carbonate system) within cyanobacterial mats, and with the intensity of in vivo biocalcification of the cyanobacteria. Silicate layers suggest that oncoids during their growth were exposed to varying external factors temporally modifying calcification. Although the thickness of silicate and carbonate layers does not depend much on their mineral composition, it seems that their formation depended on the precipitation rate within the cyanobacterial biofilm.
SEM and optical microscope observations indicate that colonies of coccoid cyanobacteria together with the filamentous ones were involved in the formation of these Bathonian oncoids. Although the coccoid cells themselves are not preserved, the presence of web-like structures, common in silicate and carbonate layers, indicates a role of coccoid cyanobacterial mats (biofilms). In our opinion, the web-like structures are the product of post-mortem decomposition and permineralization of the common mucilage sheaths (glycocalyx) that encase groups of the coccoid cyanobacterial cells that make up the mats. The sheaths display variable resistance to degradational processes and this results in the formation of the web-like structure. The thicker outer mucilage sheaths are often preserved whereas the thinner sheaths usually are degraded (e.g. Horodyski and Vonder Haar 1975; Kaźmierczak et al. 1996). Such structures are well known from modern microbialites and microbial mats, such as those described in Lake Van by Kempe et al. (1991), Kaźmierczak and Kempe (2004) and Kempe and Kaźmierczak (2007).
The filamentous structures found within oncoid coatings may represent remains of cyanobacteria, as their size and mode of preservation resemble better-preserved remnants of filamentous cyanobacteria described from other locations (Kempe et al. 1991; Kaźmierczak and Kempe 2004; Kempe and Kaźmierczak 2007; Kremer 2006). However, because of poor preservation, complete taxonomic assessment of the microbiota was not possible. Unfortunately, the organic geochemical analyses have failed to detect any biomarkers characteristic of cyanobacteria due to a very low TOC caused by a strong oxidative weathering of the studied oncoid cortices.
The enveloped oncoid cores, consisting of detrital limestones full of bio- and lithoclasts, differ lithologically from the enclosing deposits and thus look somewhat ‘exotic’, especially those within the fine siliciclastics at Blanowice. The oncoids from both localities investigated certainly underwent different formation and taphonomic histories in quite different environmental conditions, but still in the photic zone as evidenced from the coccoid cyanobacteria forming their envelopes (see also Kremer and Kaźmierczak 2005).
The cores of the Ogrodzieniec oncoids must have been eroded from pre-existing carbonate deposits. The occurrence of bioclasts of fully marine biota and ooids scattered in micritic matrix within the cores indicates that they may have originated in an environment situated at or slightly below the fair weather wavebase (corresponding to the proximal part of the mid ramp setting, see Bádenas and Aurell 2010; Olivier et al. 2011).
The provenance of the oncoid cores from Blanowice, on the other hand, may have been twofold: (1) they were eroded and transported from shallower setting (the presence of bioclasts and ooids in the cores) to the deeper site of burial, or (2) they are a remnant of a thin carbonate layer which collapsed during the liquefaction of the underlying clay sediments. Such collapse may have resulted in the formation of a carbonate clast pavement. The latter scenario has previously been suggested for similar ‘exotic’ carbonate clasts embedded within Kimmeridgian clays of central Poland by Radwański (2003), and it may well explain the occurrence of carbonate clasts within such siliciclastic setting, as well.
After deposition, the cores must have been lying on the sea floor in a calm conditions with reduced sedimentation (e.g., Peryt 1983; Gradziński et al. 2004; Reolid et al. 2005; Védrine et al. 2007) where the cyanobacterial biofilm could have developed on their upper surfaces. The residence time of the cores on the sea floor before formation of the biofilm presumably was not long, as the majority of oncoid cores are devoid of borings made by endolithozoans. Only on single specimens from Blanowice and Ogrodzieniec have the bivalve borings Gastrochaenolites been found. Episodically, however, the cores must have been repeatedly disturbed leading to their rotation and overturning which resulted in more or less concentric growth of cyanobacterial biofilm. Such disturbance could have been caused by episodic high-energy events, as storms and storm-related strong bottom currents, but biological factors as epifaunal and infaunal animals searching for food around the oncoids are possible, as well (see also Massari and Dieni 1983; Gradziński et al. 2004; Zatoń and Taylor 2009b). The presence of episodic high-energy events are best expressed in large, discoidal oncoids from Ogrodzieniec that must have been overturned as is evident from their concentric and thick microbial lamination and encrustation patterns (see below). The oncoids from Ogrodzieniec, however, were not moved repeatedly on the sea floor as the encrusting sclerobionts and the cortices themselves are not significantly abraded. Thus, the oncoids from Ogrodzieniec must have formed in an environment where sediment starvation and winnowing prevailed upon sediment influx, resulting in deposit condensation, while high energy events occurred only episodically. The latter may be demonstrated by the presence of quartz pebbles, fragmented fossils and a sandy fraction in the enclosed deposit. An environment located below the fair weather wavebase is interpreted here. Bathymetrically, it could correspond to the outer zone of the mid ramp setting of Olivier et al. (2011).
The oncoids from Blanowice, on the other hand, are interpreted to have formed in deeper, siliciclastic settings, well below the fair weather wavebase but still in the euphotic zone. Bathymetrically, It could correspond to the mid to outer ramp transitional zone of Olivier et al. (2011). The thin oncoid cortices caused by a slow down of cyanobacterial biofilm development may have resulted from both (1) lower water transparency in turbid water column, and (2) more frequent disturbance of oncoids on the sea floor, which may have been enhanced by their small-sizes. The latter may be also evident from signs of abrasion of both some encrusters and parts of the cortices. It is here interpreted that both lower luminosity and disturbance may have influenced the biofilm development. Disturbance events (probably storms) could have affected the oncoids and simultaneously resuspended the muddy bottom, causing the water column to become more turbid.
The conditions of the studied Bathonian oncoid growth was different than, for example, the Bajocian ‘snuff-boxes’ of Palmer and Wilson (1990). Although the latter oncoids formed in shallow-water settings, their microbial biofilm was interpreted to be formed in shaded conditions on the undersides of the oncoids by non-photosynthetic microorganisms.
Sedimentary conditions as evidenced from geochemical study
Generally, the composition of extracts obtained from the samples studied is comparable to most of the Middle Jurassic clays from the Polish Jura (Marynowski et al. 2007). The dominance of high molecular weight n-alkanes with an odd carbon number predominance (especially n-C25, n-C29, n-C31) indicates an input of terrestrial organic matter, typical in Middle Jurassic epicontinental basins (Marynowski et al. 2007; Hautevelle et al. 2007; Marynowski and Zatoń 2010).
Obtained values of the Pr/Ph ratio range within 1–2 (Table 1), as in the many other Middle Jurassic clay samples from Poland (Marynowski et al. 2007). These values suggest rather oxic conditions during organic matter (OM) sedimentation, but this parameter should be used with caution (for general details see Didyk et al. 1978; ten Haven et al. 1987). However, the distribution of C31 to C35 homohopanes with a significant predomination of the C31(22S+22R) epimers (Fig. 6) is characteristic of oxic conditions (Peters et al. 2005).
Fernenes most likely originated from Caytoniales, a group of Mesozoic pteridosperm plants (Zatoń et al. 2006b). The relative concentrations of fernenes, in relation to hopanes, hopenes and diasterenes, the major biomarker groups present in the clays, are rather small, but fernenes may isomerize to neohop-13(18)-ene, which is one of the most important compounds in the aliphatic fraction of the clay samples analyzed (see Fig. 6).
Quantitatively the most important aromatic compound (Table 2) is perylene, which based on new data is derived from wood-degrading fungi (Grice et al. 2009). Taking into account the large amount of wood debris within Middle Jurassic clays of the Polish Jura (Marynowski et al. 2007), such interpretation seems to be most likely.
The presence of terrestrial OM in all samples is also emphasized by the occurrence of such compounds as: dehydroabietane, simonellite and retene that are interpreted as diagenetic products of abietane type diterpenoids such as abietic acid (Marynowski et al. 2007; see also Otto and Simoneit 2001). On the other hand, cadalene, calamenene and 5,6,7,8-tetrahydrocadalene are derived from cadalene-type sesquiterpenoids, such as cadinenes and cadinols (Simoneit et al. 1986). Both groups of compounds are characteristic of conifers (Otto and Wilde 2001), but they also occur widely in the plant kingdom and are thus non-specific (Karrer et al. 1977). The other PAHs usually interpreted as pyrolytic (e.g. Marynowski and Simoneit 2009) like phenanthrene, fluoranthene, pyrene, benzo[g,h,i]fluoranthene, benz[a]anthracene, chrysene + triphenylene, benzo[e]pyrene, benzo[a]pyrene, indeno(1,2,3-cd)pyrene and benzo[ghi]perylene seem to be early diagenetic products of bacterial OM aromatization because they are present in low concentrations as compared to abundant perylene, and we have never found the strong evidences of wildfires (e.g. charcoal fragments) in the investigated clays (Marynowski et al. 2007). Despite the terrestrial OM prevalence, marine OM input onto kerogen is manifested by occurrence of the C27 diaster-13(17)-enes (20R and 20S epimers) biomarkers (for details see Marynowski et al. 2007).
Summarizing, all the samples are characterized by almost identical compositions of organic extracts (Table 1; Fig. 6). Concentrations of all compounds, biomarkers and unspecific diagenetic products (PAHs) are similar for all analyzed samples (Table 2), as well. The only differences concern the carbonate and TOC content (Table 1). This suggests that during formation of oncoids, as well as overlying carbonate concretions, the environmental conditions during sedimentation of the Bathonian clays from Blanowice were similar to those prevailing during the deposition of the Middle Jurassic ore-bearing clays of the Polish Jura area. The oncoids from Ogrodzieniec are characterized by advanced post-depositional weathering range manifested by very low TOC content due to their near-surface occurrence. In consequence they are not useful from the organic geochemical point of view.
Paleoecology of encrusting communities
Despite enormous data on paleoecology and taxonomic characteristics of encrusting organisms colonizing hard substrates, both organic and inorganic (see Taylor and Wilson 2003 for a comprehensive overview), there is a lack of detailed paleoecological studies of encrusting communities inhabiting oncoid substrates, such as are treated in this paper. As far as we know, only Palmer and Wilson (1990) presented detailed taxonomic characteristics of the biota encrusting ‘snuff-boxes’ from the Bajocian of England and France, providing the scenario of the oncoid growth and colonization patterns.
In terms of higher taxonomic rank, the encrusting communities inhabiting the Bathonian oncoids from Poland are identical to those investigated by Palmer and Wilson (1990), and very similar to the encrusting assemblages colonizing other hard substrates (e.g. Taylor and Wilson 2003). Even the microconchids (under the name Spirorbula sp.) are also present on the ‘snuff-boxes’ studied by Palmer and Wilson (1990). As with the ‘snuff-boxes’, foraminifers are also rare on the Polish oncoids. Taphonomic processes (loss due to abrasion, etc.) may be ruled out as numerous Vinelloidea foraminifers have been recently found encrusting calcitic hiatus concretions from the Polish Jura that underwent episodic transportation and abrasion (Zatoń et al. 2011). On the other hand, those hiatus concretions that preserved remnants of microbial crust simultaneously have few foraminifers (see Zatoń et al. 2011). This is interesting as it is well known that foraminifers may be abundant on such microbial substrates (see e.g., Gradziński et al. 2004; Reolid et al. 2005; Schlagintweit and Gawlick 2009). On the current stage, however, this is difficult to explain.
The entire sclerobiont fauna studied here is typical of the biota inhabiting cryptic environments, such as gloomy cavities, crevices rather than open, upward-facing surfaces. Such cryptic, or coelobiontic communities, are known from various Jurassic (e.g., Palmer and Fürsich 1974, 1981; Palmer and Wilson 1990; Fürsich et al. 1994; Wilson 1998; Taylor and Palmer 1994; Schlögl et al. 2008; Wilson et al. 2008), Cretaceous (e.g., Surlyk and Christensen 1974; Wilson 1986; Žitt and Nekvasilová 1996; Wilson and Taylor 2001) and Recent (e.g., Logan et al. 1984; Zuschin and Baal 2007; Mallela 2007) environments. Although such organisms as sabellids/serpulids may encrusted the lower and upper surfaces of the hard substrate (see e.g., Palmer and Fürsich 1974; Zuschin and Baal 2007), they are most abundant on the undersides, anyway. However, such encrusters as thecideide brachiopods, sclerosponges, bryozoans and scleractinian corals are known to occur (but it is not a rule; see e.g., Wilson et al. 2008 for some sponges and corals) on the undersides, in crevices, and other gloomy places (see e.g., Kaźmierczak and Hilmer 1974; Palmer and Fürsich 1974; Taylor and Palmer 1994; Zuschin and Baal 2007; Schlögl et al. 2008; Krawczyński 2008). Though, in the case of mobile substrates, such as the oncoids studied here, it is difficult to differentiate between the open upper and hidden lower surfaces. The organisms mentioned above are found to encrust such places of the oncoids as their lateral sides, various recesses (concavities) in the cortex, or in the shadow of larger encrusters like worm tubes (as in the case of small thecideide brachiopods). Also, microconchids are exclusively found on the lateral ‘vertical’ parts of the oncoids and their concavities, which emphasizes the cryptic nature of these extinct encrusters. Also the oncoids from Ogrodzieniec, although generally flattened in shape, possessed available substrate for colonization of their undersides. This was determined by the shape of the inner cores of the oncoids that very often show their convexities both on the upper and lower sides, and thus providing a sort of lifter of the oncoids off the sea floor.
It is clear that all the assemblages of these cryptic encrusters are dominated by sabellid and serpulid worms (Table 3), as in the case of the oncoids studied by Palmer and Wilson (1990). The other groups, except the unidentifiable cyclostome bryozoans ‘Berenicea’, occur in much lower numbers in relation to the above-mentioned ‘worms’ (Table 3). It may have resulted from competitive interaction with dominant sabellids and serpulids and/or much higher fecundity of the latter organisms. The rarity of microconchids may be caused by their low competitive abilities for both space and food gathering with other dominant encrusters and also by their overall decline in the Middle Jurassic, as the last representatives are known from the Upper Bathonian (see Vinn and Taylor 2007; Vinn and Mutvei 2009; Vinn 2010). The sclerobionts could have colonised the biofilm very quickly after its formation, before it became lithified, as such organisms can encrust a non-lithified (firm) substrate as well (see Taylor and Wilson 2003).
Comparing the other mobile substrates from the Bajocian and Bathonian of the Polish Jura, namely the hiatus concretions (see Zatoń et al. 2006a, 2011), the diversity of encrusters inhabiting the Ogrodzieniec oncoids studied here seems to be quite similar, attaining at least 26 taxa. The hiatus concretions, however, possess a lower generic diversity of sabellids and serpulids and completely lack thecideide brachiopods and microconchids (Zatoń et al. 2011). It may have resulted from intensity of disturbance, as the borings and encrusters from the hiatus concretions are very often abraded. This, in contrast to the oncoids, may point to a more intensive hydrological regime and/or instability of smaller hiatus concretions that promoted their transport and subsequent abrasion after colonization.
The high encruster diversity and abundance noted on the oncoids from Ogrodzieniec, their formation by the light-dependent cyanobacteria, as well as the diverse fauna in the enclosing carbonate deposits may point to a normal-marine environment with transparent waters and probably meso- to eutrophic conditions (e.g., Lescinsky et al. 2002). In the case of the Blanowice oncoids occurring in a siliciclastic setting, where sedentary polychaetes dominate over the otherwise poorly diversified encrusting assemblage, the trophic conditions may have been worse. This, and the poorly developed cortex lamination, may indicate mesotrophic conditions in marine waters with lower transparency.
Any borings made by bivalves or worms, which are common on calcareous hard substrates, including the hiatus concretion from the Polish Jura mentioned above, have not been found on the oncoid cortices investigated. The same concerns the oncoids from the Bajocian of England and France (see Palmer and Wilson 1990). The bivalve borings have only been found in some of the inner cores of the oncoids from Blanowice and Ogrodzieniec. This may imply that in the present case borers preferred only the calcareous hard substrate and not the microbial biofilm enveloping it (see also Palmer and Wilson 1990). Palmer and Wilson (1990) stated that boring organisms were unable to bore through the ferruginous crust of their ‘snuff-boxes’. A similar situation may have taken place in the case of the Polish Jura oncoid cortices, as they are composed of alternating carbonate and silicate laminae. The boring biota may have simply not been able to bore through the silicates. Alternatively, the biofilm itself may have been too soft for borers. It must be noted, however, that borings (Trypanites) have been noted in other microbialites by Olivier et al. (2011).
Conclusions
The Middle Jurassic oncoids from Ogrodzieniec and Blanowice (Polish Jura), although similar in age (Bathonian), differ with respect to their sedimentological context, morphology and size, as well as their paleontological content. The oncoids from Ogrodzieniec occur within carbonate bed, and those from Blanowice are embedded within fine siliciclastics. The oncoids from both Ogrodzieniec and Blanowice originated in fully marine environments. The oncoids from Ogrodzieniec are interpreted to have formed in a calm environment below the fair weather wavebase where sediment starvation prevailed over sediment influx. The oncoids from Blanowice, on the other hand, originated in a deeper, muddy-bottom environment, well below the fair weather wavebase.
Resting on the sea floor during periods of reduced sedimentation and episodic overturning, the cores were then coated with laminated cortices on their surfaces. From the striking morphological and mineralogical similarity between the web-like structures occurring in the Bathonian oncoid cortices and in some modern cyanobacterial microbialites, it can be concluded that the studied oncoids originated from communities of benthic coccoid cyanobacteria with some addition of filamentous cyanobacteria. Thus, it is interpreted that the oncoids from both localities investigated formed within the photic zone. However, those from Blanowice were affected by more turbid waters and probably higher disturbance, which is evidenced in their undeveloped, thin cortex lamination and partial abrasion, as compared to the oncoids from Ogrodzieniec, which have thick and well-developed cortex.
The sedimentary conditions during oncoid cortices formation were oxic, what is also emphasized by lower TOC content as compared to host clays, high Pr/Ph ratio and significant predomination of the C31 homohopanes. This may be connected with longer exposition of the oncoids on the sea floor, but in general, their biomarker characteristics is similar to those occurring during the deposition of the Middle Jurassic ore-bearing clays of the Polish Jura area.
Along with biofilm formation, various encrusting organisms settled on the oncoids, the majority of which are cryptic. The whole encrusting assemblages are dominated by sabellids/serpulids and bryozoans, with an addition of foraminifers, calcisponges, corals, bivalves, microconchids and brachiopods. The much greater abundance and diversity of encrusters in the Ogrodzieniec oncoids may point to better trophic conditions during their formation than in the case of the Blanowice oncoids. In total, both the Blanowice and Ogrodzieniec oncoids hosted at least 26 taxa, which makes them the most diverse oncoid encruster community ever reported.
References
Ageta H, Arai Y (1983) Fern constituents: pentacyclic triterpenoids isolated from Polypodium niponicum and P. formosanum. Phytochem 22:1801–1808
Alexander R, Bastow TP, Fisher SJ, Kagi RI (1995) Geosynthesis of organic compounds: II. Methylation of phenanthrene and alkylphenanthrene. Geochim Cosmochim Acta 59:4259–4266
Aurell M, Fernández-López S, Melendez G (1994) The Middle-Upper Jurassic oolitic ironstone level in the Iberian range (Spain). Eustatic implications. Geobios. Mem Spec 17:549–561
Bádenas B, Aurell M (2010) Facies models of a shallow-water carbonate ramp based on distribution of non-skeletal grains (Kimmeridgian, Spain). Facies 56:89–110
Brand U, Veizer J (1983) Origin of coated grains: trace element constraints. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 9–26
Burkhalter RM (1995) Ooidal ironstones and ferruginous microbialites: origin and relation to sequence stratigraphy (Aalenian and Bajocian, Swiss Jura Mountains). Sedimentol 42:57–74
Dayczak-Calikowska K, Kopik J, Marcinkiewicz T (1997) Middle Jurassic. In: Marek S, Pajchlowa M (eds) Epikontynentalny perm i mezozoik w Polsce. Pr Państ Inst Geol 153: 236–282
Didyk BM, Simoneit BRT, Brassell SC, Eglinton G (1978) Organic geochemical indicators of palaeoenvironmental conditions of sedimentation. Nature 272:216–222
Dromart G, Gaillard C, Jansa LF (1994) Deep-marine microbial structures in the Upper Jurassic of western Tethys. In: Bertrand-Sarfati J, Monty C (ed) Phanerozoic stromatolites II. Kluwer Academic Publishers, Dordrecht, pp 295–318
Flügel E (2004) Microfacies of carbonate rocks–analysis, interpretation and application. Springer, Berlin Heidelberg New York, p 976
Fürsich FT, Palmer TJ, Goodyear KL (1994) Growth and disintegration of bivalve-dominated patch reefs in the upper Jurassic of southern England. Palaeontol 37:131–171
Gatrall M, Jenkyns HC, Parsons CF (1972) Limonitic concretions from the European Jurassic, with particular reference to the “snuff-boxes” of southern England. Sedimentol 18:79–103
Giżejewska M, Wieczorek J (1976) Remarks on the Callovian and lower Oxfordian of the Zalas Area (Cracow Upland, Southern Poland). Bull Acad Polon Sci, Ser Sci Terre 24:167–175
Gradziński M, Tyszka J, Uchman A, Jach R (2004) Large microbial-foraminiferal oncoids from condensed Lower-Middle Jurassic deposits: a case study from the Tatra Mountains, Poland. Palaeogeog Palaeoclim Palaeoecol 213:133–151
Grice K, Lu H, Atahan P et al (2009) New insights into the origin of perylene in geological samples. Geochim Cosmochim Acta 73:6531–6543
Hautevelle Y, Michels R, Malartre F, Elie M, Trouiller A (2007) Tracing of variabilities within a geological barrier by molecular organic geochemistry: case of the Callovo-Oxfordian sedimentary series in the East of the Paris Basin (France). Appl Geochem 22:736–759
Herbig HG (1994) Cyanoide und Stromatolithe aus dem marokkanischen Alttertiär. Abh Geol BA 50:155–167
Horodyski RJ, Vonder Haar SP (1975) Recent calcareous stromatolites from Laguna Mormona (Baja California) Mexico. J Sediment Petrol 45:894–906
Ippolitov AP (2007a) Contribution to the revision of some Late Callovian serpulids (Annelida, Polychaetes) of Central Russia: part 2. Paleontol J 41:260–267
Ippolitov AP (2007b) Contribution to the revision of some Late Callovian serpulids (Annelida, Polychaetes) of Central Russia: part 1. Paleontol J 41:429–436
Karrer W, Cherbuliez E, Eugster CH (1977) Konstitution und Vorkommen der organischen Pflanzenstoffe (exclusive Alkaloide). Ergänzungsband 1. Birkhäuser, Basel
Kaźmierczak J, Hilmer G (1974) Sclerosponge nature of the Lower Hauterivian “Bryozoan” Neuropora pustulosa (Roemer, 1839) from Western Germany. Acta Palaeontol Polon 19:443–453
Kaźmierczak J, Kempe S (2004) Microbialite formation in seawater of increased alkalinity, Satonda Crater Lake, Indonesia–Discussion. J Sediment Res 74:314–317
Kaźmierczak J, Coleman ML, Gruszczynski M, Kempe S (1996) Cyanobacterial key to the genesis of micritic and peloidal limestones in ancient seas. Acta Paleontol Polon 41:319–338
Kempe S, Kaźmierczak J (2007) Hydrochemical key to the genesis of calcareous nonlaminated and laminated cyanobacterial microbialites. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments. Springer, Berlin Heidelberg New York, pp 241–264
Kempe S, Kaźmierczak J, Landmann G et al (1991) Largest known microbialites discovered in Lake Van, Turkey. Nature 394:605–608
Kopik J (1998) Jura dolna i środkowa północno-wschodniego obrzeżenia Górnosląskiego Zagłębia Węglowego. Biul Państw Inst Geol 378:67–120
Krawczyński C (2008) The Upper Oxfordian (Jurassic) thecideide brachiopods from the Kujawy area, Poland. Acta Geol Polon 58:395–406
Kremer B (2006) Mat-forming coccoid cyanobacteria from early Silurian marine deposits of Sudetes, Poland. Acta Palaeontol Polon 51:143–154
Kremer B, Kaźmierczak J (2005) Cyanobacterial mats from Silurian black radiolarian cherts: phototrophic life at the edge of darkness? J Sediment Res 75:897–906
Lescinsky HL, Edinger E, Risk MJ (2002) Mollusc shell encrustation and bioerosion rates in a modern epeiric sea: taphonomy experiments in the Java Sea, Indonesia. Palaios 17:171–191
Logan A, Mathers SM, Thomas MLH (1984) Sessile invertebrate coelobite communities from reefs of Bermuda: species composition and distribution. Coral Reefs 2:205–213
Majewski W (2000) Middle Jurassic concretions from Częstochowa (Poland) as indicators of sedimentation rates. Acta Geol Polon 50:431–439
Mallela J (2007) Coral reef encruster communities and carbonate production in cryptic and exposed coral reef habitats along a gradient of terrestrial disturbance. Coral Reefs 26:775–785
Marynowski L, Simoneit BRT (2009) Widespread Late Triassic to Early Jurassic wildfire records from Poland: evidence from charcoal and pyrolytic polycyclic aromatic hydrocarbons. Palaios 24:785–798
Marynowski L, Zatoń M (2010) Organic matter from the Callovian (Middle Jurassic) deposits of Lithuania: compositions, sources and depositional environments. Appl Geochem 25:933–946
Marynowski L, Zatoń M, Simoneit BRT et al (2007) Compositions, sources and depositional environments of organic matter from the Middle Jurassic clays of Poland. Appl Geochem 22:2456–2485
Massari F, Dieni I (1983) Pelagic oncoids and ooids in the Middle-Upper Jurassic of eastern Sardinia. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 367–408
Matyja BA, Wierzbowski A (2000) Ammonites and stratigraphy of the uppermost Bajocian and Lower Bathonian between Częstochowa and Wieluń, Central Poland. Acta Geol Polon 50:191–209
Matyja BA, Wierzbowski A, Gedl P et al. (2006a) Stop B1.5—Sowa’s and Glinski’s clay pits (uppermost Bajocian-lowermost Bathonian). In: Wierzbowski A, Aubrecht R, Golonka J et al. (eds) Jurassic of Poland and adjacent Slovakian Carpathians. Field trip guidebook of 7th International Congress on the Jurassic system Poland, Kraków, September 6-18, 2006, pp 149–152
Matyja BA, Wierzbowski A, Gedl P et al. (2006b) Stop B1.6—Leszczyński’s clay pit (Lower Bathonian). In: Wierzbowski A, Aubrecht R, Golonka J et al. (eds) Jurassic of Poland and adjacent Slovakian Carpathians. Field trip guidebook of 7th International Congress on the Jurassic system Poland, Kraków, September 6–18, 2006, pp 152–154
Matyja BA, Wierzbowski A, Gedl P et al. (2006c) Stop B1.7—Gnaszyn clay pit (Middle Bathonian-lowermost Upper Bathonian). In: Wierzbowski A, Aubrecht R, Golonka J et al. (eds) Jurassic of Poland and adjacent Slovakian Carpathians. Field trip guidebook of 7th International Congress on the Jurassic System Poland, Kraków, September 6–18, 2006, pp 154–157
Nickel E (1983) Environmental significance of freshwater oncoids, Eocene Guanga Formation, southern Pyrenees, Spain. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 308–329
Olivier N, Colombie C, Pittet B, Lathuiliere B (2011) Microbial carbonates and corals on the marginal French Jura platform (Late Oxfordian, Molinges section). Facies (in press) doi:10.1007/s10347-010-0246-9
Otto A, Simoneit BRT (2001) Chemosystematics and diagenesis of terpenoids in fossil conifer species and sediment from the Eocene Zeitz formation, Saxony, Germany. Geochim Cosmochim Acta 65:3505–3527
Otto A, Wilde V (2001) Sesqui-, di-, and triterpenoids as chemosystematic markers in extant conifers–a review. Botanic Rev 67:141–238
Palmer TJ, Fürsich FT (1974) The ecology of a Middle Jurassic hardground and crevice fauna. Palaeontol 17:507–524
Palmer TJ, Fürsich FT (1981) Ecology of sponge reefs from the Upper Bathonian of Normandy. Palaeontol 24:1–23
Palmer TJ, Wilson MA (1990) Growth of ferruginous oncoliths in the Bajocian (Middle Jurassic) of Europe. Terra Nova 2:142–147
Paull R, Michaelsen BH, McKirdy DM (1998) Fernenes and other terpenoid hydrocarbons in Dicroidium-bearing Triassic mudstones and coals from South Australia. Org Geochem 29:1331–1343
Peryt TM (1981) Phanerozoic oncoids–an overview. Facies 4:197–214
Peryt TM (1983) Classification of coated grains. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 3–6
Peters KE, Walters CC, Moldowan JM (2005) The biomarker guide. Vol. 2, Cambridge University Press, Cambridge
Pillai TG (1993) A review of some Cretaceous and Tertiary serpulid polychaetes of the genera Cementula and Spiraserpula Regenhardt 1961, Laqueoserpula Lommerzheim 1979 and Protectoconorca Jager 1983. Paläont Z 67:69–88
Radwańska U (2004) Tube-dwelling polychaetes from the upper Oxfordian of Wapienno/Bielawy, Couiavia region, north-central Poland. Acta Geol Polon 54:35–52
Radwański A (2003) Rezidualne brykiety kolapsyjne w pakietach margli górnego kimerydu Małogoszcza. Tomy Jurajskie 1:77–83
Reolid M, Nieto LM (2010) Jurassic Fe-Mn macro-oncoids from pelagic swells of the External Subbetic (Spain): evidences of microbial origin. Geol Acta 8:151–168
Reolid M, Gaillard C, Olóriz F, Rodríguez-Tovar FJ (2005) Microbial encrustations from the Middle Oxfordian-earliest Kimmeridgian lithofacies in the Prebetic Zone (Betic Cordillera, southern Spain): characterization, distribution and controlling factors. Facies 50:529–543
Reolid M, Nieto LM, Rey J (2010) Taphonomy of cephalopod assemblages from Middle Jurassic hardgrounds of pelagic swells (South-Iberian Palaeomargin, Western Tethys). Palaeogeog Palaeoclimat Palaeoecol 292:257–271
Riding R (1983) Cyanoliths (cyanoids): oncoids formed by calcified cyanophytes. In: Peryt TM (ed) Coated grains. Springer, Berlin Heidelberg New York, pp 276–283
Różycki SZ (1953) Górny dogger i dolny malm Jury Krakowsko-Częstochowskiej. Pr Inst Geol 17:1–420
Schlagintweit F, Gawlick H-J (2009) Oncoid-dwelling foraminifera from Late Jurassic shallow-water carbonates of the Northern Calcareous Alps (Austria and Germany). Facies 55:259–266
Schlögl J, Michalík J, Zágoršek K, Atrops F (2008) Early Tithonian serpulid-dominated cavity-dwelling fauna, and the recruitment pattern of the serpulid larvae. J Paleontol 82:382–392
Simoneit BRT, Grimalt JO, Wang TG et al (1986) Cyclic terpenoids of contemporary resinous plant detritus and of fossil woods, amber and coal. Org Geochem 10:877–889
Surlyk F, Christensen WK (1974) Epifaunal zonation on an upper Cretaceous rocky coast. Geology 2:529–534
Szulczewski M (1968) Jurassic stromatolites of Poland. Acta Geol Polon 18:1–96
Taylor PD, Ernst A (2008) Bryozoans in transition: the depauperate and patchy Jurassic biota. Palaeogeog Palaeoclimat Palaeoecol 263:9–23
Taylor PD, Palmer TJ (1994) Submarine caves in a Jurassic reef (La Rochelle, France) and the evolution of cave biotas. Naturwissenshaften 81:357–360
Taylor PD, Sequeiros L (1982) Toarcian bryozoans from Belchite in north-east Spain. Bull Brit Mus (Nat Hist) Geol Ser 37:117–129
Taylor PD, Wilson MA (2002) A new terminology for marine organisms inhabiting hard substrates. Palaios 17:522–525
Taylor PD, Wilson MA (2003) Palaeoecology and evolution of marine hard substrate communities. Earth-Sci Rev 62:1–103
ten Haven HL, de Leeuw JW, Rullkötter J, Sinninghe Damsté JS (1987) Restricted utility of the pristane/phytane ratio as a palaeoenvironmental indicator. Nature 330:641–643
Tucker ME, Wright VP (1990) Carbonate sedimentology. Oxford, United Kingdom, Blackwell Scientific Publications, p 482
Védrine S, Strasser A, Hug W (2007) Oncoid growth and distribution controlled by sea-level fluctuations and climate (Late Oxfordian, Swiss Jura Mountains). Facies 53:535–552
Vinn O (2010) Adaptive strategies in the evolution of encrusting tentaculitoid tubeworms. Palaeogeog Palaeoclimat Palaeoecol 292:211–221
Vinn O, Mutvei H (2009) Calcareous tubeworms of the Phanerozoic. Eston J Earth Sci 58:286–296
Vinn O, Taylor PD (2007) Microconchid tubeworms from the Jurassic of England and France. Acta Palaeontol Polon 52:391–399
Wilson MA (1986) Coelobites and spatial refuges in a lower Cretaceous cobble-dwelling hardground fauna. Palaeontol 29:691–703
Wilson MA (1998) Succession in a Jurassic marine cavity community and the evolution of cryptic marine faunas. Geology 26:379–381
Wilson MA, Taylor PD (2001) Palaeoecology of hard substrate faunas from the Cretaceous Qahlah Formation of the Oman Mountains. Palaeontol 44:21–41
Wilson MA, Feldman HR, Bowen JC, Avni Y (2008) A new equatorial, very shallow marine sclerozoan fauna from the Middle Jurassic (late Callovian) of southern Israel. Palaeogeog Palaeoclimat Palaeoecol 263:24–29
Zatoń M (2007) Tulites cadus Buckman, 1921 (Ammonoidea) from the Middle Bathonian of the Polish Jura and its biostratigraphic significance. N Jahrb Geol Pal Abh 243:191–199
Zatoń M, Taylor PD (2009a) Middle Jurassic cyclostome bryozoans from the Polish Jura. Acta Palaeontol Polon 54:267–288
Zatoń M, Taylor PD (2009b) Microconchids (Tentaculita) from the Middle Jurassic of Poland. Bull Geosci 84:653–660
Zatoń M, Taylor PD (2010) Bathonian (Middle Jurassic) cyclostome bryozoans from the Polish Jura. Bull Geosci 85:275–302
Zatoń M, Marynowski L, Bzowska G (2006a) Konkrecje hiatusowe z iłów rudonośnych Wyżyny Krakowsko-Częstochowskiej. Przeg Geol 54:131–138
Zatoń M, Barbacka M, Marynowski L, Krzystanek J (2006b) Sagenopteris (Caytoniales) with its possible preserved biomarkers from the Bathonian of the Polish Jura, south-central Poland. N Jahrb Geol Pal Monat 7:385–402
Zatoń M, Marynowski L, Szczepanik P et al (2009) Redox conditions during sedimentation of the Middle Jurassic (Upper Bajocian-Bathonian) clays of the Polish Jura (south-central Poland). Facies 55:103–114
Zatoń M, Machocka S, Wilson MA, Marynowski L, Taylor PD (2011) Origin and paleoecology of Middle Jurassic hiatus concretions from Poland. Facies 57:275–300 doi:10.1007/s10347-010-0244-y
Žitt J, Nekvasilová O (1996) Epibionts, their hard-rock substrates, and phosphogenesis during the Cenomanian-Turonian boundary interval (Bohemian Cretaceous Basin, Czech Republic). Cret Res 17:715–739
Zuschin M, Baal C (2007) Large gryphaeid oysters as habitats for numerous sclerobionts: a case study from the northern Red Sea. Facies 53:319–327
Acknowledgments
We thank our friends Czarek Krawczyński (Museum of the Earth, Warsaw) and Elek Szełęg (Faculty of Earth Sciences, Sosnowiec) for their help concerning the thecideide brachiopod identification and mineralogical observations. Felix Schlagintweit and Nicolas Olivier, the journal reviewers, made many useful remarks and comments which are greatly appreciated. André Freiwald, the journal editor, is thanked for his help with editorial matters. The study was conducted under the GeoBio-Center of LMU Munich, Germany (BK).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zatoń, M., Kremer, B., Marynowski, L. et al. Middle Jurassic (Bathonian) encrusted oncoids from the Polish Jura, southern Poland. Facies 58, 57–77 (2012). https://doi.org/10.1007/s10347-011-0273-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10347-011-0273-1