Abstract
As one cause for biodiversity loss, invasive alien species are a worldwide threat. In forests, however, invasive tree species can also have an enormous biomass potential which can be harvested while taking measures against the species. Allometric equations help estimating the biomass but are often only available for the native range of the species. This lack on information complicates the management of invaded stands, and the equations presented here should help fill this gap. The above-ground biomass for single trees of black cherry (Prunus serotina Ehrh.) and black locust (Robinia pseudoacacia L.) in Ticino/Italy was estimated with differing explanatory variables as total, stem, crown, and leaf biomass. Regression equations of P. serotina were compared with equations from North America. The methods to derive biomass estimates from fresh weight and volumetric measurements in combination with wood densities were critically examined. The biomass could be estimated well by using “diameter” as explanatory variable. The productivity of P. serotina was lower here compared to its range of origin. Biomass estimates from volumetric measurements combined with the truncated cone formula have lead to systematic overestimations. Also the use of volumetric measurements combined with wood density measurements has overestimated comparable estimates from fresh weight measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien species continue to be a threat to ecosystems especially as a cause for biodiversity loss and thus possibly modifying ecological key processes through replacement (Closset-Kopp et al. 2007; Higgins et al. 1999; Mooney 1999; Nentwig et al. 2005; Vitousek et al. 1996; Williamson 1999). Additionally to land-use change, it is even widely accepted that biological invasions are one of the leading causes of biodiversity loss worldwide (Chabrerie et al. 2008; Chapin et al. 2000; Cronk and Fuller 1995; MA 2005; Sax and Gaines 2003; Solbrig 1991). Against this background, monitoring invasive species, preventing their further spread, and finally, diminishing or even exterminating populations of invasive alien species, is necessary and particularly in cases where ecosystem services are negatively affected.
In Europe, black cherry (Prunus serotina Ehrh.) and also black locust (Robinia pseudoacacia L.) to which the cherry is compared in this paper are alien species, both introduced to Europe in the seventeenth century (Roloff et al. 1994; Wein 1930, 1931) and both invasive on many sites. For example, P. serotina can replace native plant species and communities, thus being a threat to native forests (Starfinger 1991). R. pseudoacacia is mainly a threat for the identity and integrity of nutrient-poor sites on which its ability to fix nitrogen with help of symbiosis can cause an unwanted and long-lasting shift in vegetation composition toward nitrogen-rich and species-poor plant communities (Chapman 1935; Göhre 1952; Jurko 1963; Kowarik 2010). Once established, both species can cause severe management problems (Brehm 2004; Roloff et al. 1994).
However, in the case of P. serotina, it seems impossible to completely eradicate the species in Europe at reasonable cost or measures and imagine Central European plant communities free of this species. Without surrendering to P. serotina, it might be time to not only think about how to proceed against it (e.g., Brehm 2004; Brosemann and Krug 2006), but also consider it as biomass-producing plant and study its productivity. This also holds true against the background of globally discussed use of biomass for energy production (e.g., Burwell 1978; Hall et al. 1991; Ohlrogge et al. 2009; Rostrup-Nielsen 2005). Furthermore, the impact of an invader on a community is expected to correlate with the population density of the invader, since any space or resource controlled by the invader constitutes resources no longer available to the other species (Chabrerie et al. 2008; Parker et al. 1999). Consequently, the extent of the impact depends also on the total biomass of the species. Extracting biomass could hence reduce the impact and keep the invader from functioning as “invasive engineer” (Cuddington and Hastings 2004), “strong invader” (Ortega and Pearson 2005), or “transformer” (Richardson et al. 2000) by changing the species composition and ecosystem function (Brown and Peet 2003; Chabrerie et al. 2008).
Furthermore, pruning the species by using its biomass would not only reduce its dominance but eventually exhaust the species abundance. The biomass harvested till then could also pay for parts of the measures taken against the species.
In the biosphere reserve “Valle del Ticino” in Northern Italy, P. serotina is invasive by dominating regeneration and understory of the forests and has prevented a successful reproduction of the tree species native to the region so far (e.g., Quercus robur, Carpinus betulus, Ulmus sp.). R. pseudoacacia also occurs in the reserve but is not considered to be invasive here and only has a comparative purpose in this study as other alien species. The riverside biosphere reserve still encompasses a mosaic of ecosystems typical of large rivers like wetlands, riparian forests, and large river habitats. In sensitive ecosystems, broad-spectrum herbicides (e.g., glyphosate) to control P. serotina can be questioned, especially where a targeted application renders difficulties due to water dynamics, whereas a regular cut back, harvest, and plantings against P. serotina might be a more environment-friendly measure.
In the region, selective cuttings to prevent a further spread of P. serotina have been conducted for exactly these reasons. However, for estimating the potential yield which is needed for economic considerations, it is essential to estimate the above-ground biomass of P. serotina. We have to face the knowledge gap of biomass functions for P. serotina growing in Europe.
Consequently, we want to test the hypotheses that (a) P. serotina has a different biomass potential in Europe compared to its native range of origin because of the differing abiotic conditions and we also want to discuss (b) the method of using wood densities to estimate the AGB biomass which can result in false estimations.
The objectives of this study are therefore (1) deriving biomass functions and studying the productivity (AGB and ρ) of P. serotina and additionally for R. pseudoacacia as other alien tree species growing in the Po plain for comparative purposes (2) comparing the European growth performance of P. serotina with biomass equations from their native range in North America, and (3) comparing methods to estimate dry weight from fresh weight and volumetric measurements.
Materials and methods
Study area
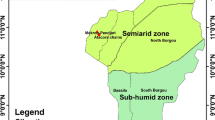
The study was conducted in the biosphere reserve “Valle del Ticino.” The reserve is located in Northwest Italy near the city of Milano and covers an area of about 971.4 km2 (Fig. 1). The riverside reserve follows the Ticino river from its outlet at Lake Maggiore in the North (45°06′N–45°46′N) into the region of Lombardy, until the Ticino river joins the Po river south of Pavia (08°34′E–09°16′E). The park has a length of more than 80 km and a width between 5 and 15 km with an altitude of about 50–250 m above sea level. In 2002, the park was acknowledged as part of the “UNESCO Man and Biosphere Programme” as MAB Biosphere Reserve “Valle del Ticino” (UNESCO 2005).
The climate of the park is related to its geographical location in the Po Valley. The valley is surrounded by the Alps in the north and west and the Apennines mountains in the south and is only open to the sea on the Adriatic coast in the east. Because of this geographical situation, remarkable frosts can occur in winter whereas heat can build up in summer. So unlike the rest of Italy, the climate of the Po Valley is not Mediterranean, but more temperate sub-continental (Ferré et al. 2005). It is humid with an average annual temperature of about 13 °C and most of the rain falling in spring and autumn with an average annual precipitation of up to 980 mm. The soil in the area of the study site can be considered as strongly acid with a pH range from 4.41 to 5.38 with a mean pH value of about 5 (measured in Aqua dest.) but a quite low C/N ratio of around 14.5.
As typical for floodplains, the vegetation is azonal and influenced more by the river (and anthropogenic activities) than the climatic conditions. The core zone of the park consists mainly of riparian woodland but also includes scattered plantations of hybrid poplar (Populus × canadensis). In floodplain forests along rivers, the woodland can be differentiated into softwood and hardwood forests. Our study focuses on the latter. Pedunculate oak (Q. robur) with Solomon’s seal (Polygonatum odoratum) are representatives for the plant associations currently existing here as the Polygonato multiflori-Quercetum roboris (Minelli et al. 2002). In the early twentieth century, various alien tree species (e.g., P. serotina, R. pseudoacacia, Quercus rubra) populated the park area. Presumably, they have migrated from gardens of nearby cities, like Gallarate (Fig. 1) in the north in the case of P. serotina or from plantations and other human activities in the region. At present, R. pseudoacacia and Q. rubra (Red Oak) can be found throughout the park. P. serotina has not yet gone far beyond the southern border of the city Abbiategrasso (Fig. 1).
The trees harvested for the development of biomass functions all originate from the woodland “Boschi di Castelletto” (Fig. 1), which is an extensive forest area originally mainly consisting of Q. robur with some specimen of C. betulus and Corylus avellana but now strongly infiltrated by P. serotina and also R. pseudoacacia. This forest is appropriate for deriving biomass functions representative for local hardwood forests because the conditions are similar to those which can be found in other hardwood forests of the region, invaded or not as strongly invaded so far. At the same time, these forests represent one of the most widely occurring forest types of the hardwood forest in the Ticino valley.
Field sampling and measurements
A total of n = 95 trees were harvested as a whole and used to derive the biomass functions presented here of which n = 82 were P. serotina trees and n = 13 were R. pseudoacacia trees. The differing sampling size among species was related to the time of harvest and our focus on P. serotina.
The data for P. serotina were collected during two different felling activities conducted in the area in April 2010 (sample 1, n = 35) and February 2011 (sample 2, n = 47). The data for R. pseudoacacia were only collected during felling activities in February 2011. This felling was conducted during a preventive cutting against P. serotina. Here, R. pseudoacacia functioned as one competitor among others of P. serotina and was to be spared as far as possible. For this reason, we have concentrated on trying to cover the dbh (diameter at breast height) range but had to restrict ourselves to a small amount of individuals in each dbh class of R. pseudoacacia.
The following criteria were used to select the sample trees for the biomass analysis: (1) species (P. serotina or R. pseudoacacia), (2) vitality (visual vitality criteria like pathogen infestation and major stem and top ruptures), (3) dbh (diameter at breast height) or d_0.1 (diameter 10 cm above ground), and (4) growth type (root suckers and coppice sprouts vs. plants originated generatively from seeds). Species, vitality, and growth type were determined visually. dbh was measured in cm at a height of 1.3 m over bark using a calliper or girthing tape. d_0.1 was only recorded for P. serotina trees with a dbh < 7 cm and was measured over bark using a calliper with an accuracy of a tenth of a millimeter. The reason to measure d_0.1 was that for some smaller trees no dbh could be measured, for example, when trees were <1.3 m or when there was no clear trunk axis in 1.3 m height.
Finally, the selected trees were vital with no visual pathogen infestation or major top ruptures or other damages, distributed quite evenly over the diameter range from about 1 cm (d_0.1) to almost 36 cm (dbh) with a height of up to 17.5 m and of generative origin as far as could be determined (Table 1). We tried to cover the dbh range as far as possible. During local forest inventories, a maximum dbh of 38.6 cm for P. serotina and 26 cm for R. pseudoacacia and heights of 22.4 and 20.5 m were found, respectively. We decided to not include vegetative growth since it is quite variable depending on the stock it originates from.
All selected trees were harvested as data basis for the biomass functions. Trees were felled as close to ground as possible. In cases in which it was not possible to cut close to ground, the remaining tree stump was recorded volumetrically measuring bottom and top diameter and length to be able to estimate its biomass later. The measurements of length, bottom, and top diameter were used in the truncated cone formula to calculate the volume (VOL) of the remaining part by using Eq. 1 where L is length (cm), r b is bottom radius (cm) and r t is top radius (cm):
The VOL was later converted to dry weight (DW) biomass (cp. 2.3). Volumetric measurements like described here were also used by Brown (1997) to estimate the above-ground biomass (AGB) per unit of green VOL for plant parts, which were too voluminous or heavy to be weighed under field conditions.
After felling, the trees were separated into the categories crown and stem and measurements of total height, crown, and stem length were added to the diameter measurements. The beginning of the crown was defined as where a straight trunk axis could not be recognized any longer. All wood following this point was assigned to the crown biomass. Topping off at a certain top diameter, like done for coniferous species, was not possible for these species, as a result of their sympodial growth and lack of apical dominance. Also the diameter range would have complicated this. Trees harvested in 2010 were already carrying leaves, so these were added as third category for those trees. For this purpose, all leaves were stripped from the branches manually and separated from the woody tree parts.
Finally, the fresh weight (FW) of each category (crown, stem, leaf) was measured directly in the field using a scale. For this purpose, the woody parts were cut into smaller logs. Parts of the stem that were too heavy to scale were divided into straight regularly shaped sections and measured volumetrically like explained above for remaining tree stumps. To calibrate and control the conversions from VOL to DW, we conducted additional volumetric measurements of other regularly shaped sections, which, however, could also be weighed in field.
For later conversion of VOL and FW to DW, tree sub-samples for laboratory measurements were collected from the sample trees as wood samples from stem (n Prunus = 101, n Robinia = 20) and crown (n Prunus = 32, n Robinia = 8) and as leaf samples (n Prunus = 29). The leaf samples were taken in equal weight proportions from the lower and upper half of the trees. Each sample consisted of a varying amount of leaves. For the wood density estimates, disks were taken in different heights along the tree axis at a distance of 1 m, respectively, and from the crown in decreasing diameter classes. Their FW was measured directly in the field with an accuracy of 1 g.
Lab measurements
In order to determine DW, we used samples brought from the field for which fresh weight measurements had been conducted in the field (cp. 2.2). These samples from stem, crown, and leaves were dried at 105 °C in a temperature-controlled oven at ambient pressure until a constant weight was achieved (DW). After 72 h, this was the case for the woody parts of P. serotina, the samples from R. pseudoacacia remained in the oven twice as long. The leaves were in the oven for 48 h. All samples were then weighed again with an accuracy of one gram. From these measurements, the relation of FW to DW (FW∝DW) could be calculated for each category after testing for significant differences between individual trees, sampling season, and sample position concerning the woody samples collected from crown or stem.
For converting the VOL measurements into DW, we measured the volume of stem disk samples (n Prunus = 81, n Robinia = 20) and crown samples (n Prunus = 25, n Robinia = 8) by immersing them in a container filled with water and measuring the amount of water displaced, like described as water displacement method by Zobel and Buijtenen (1989), Correia et al. (2010), and Tabacchi et al. (2011). These volume measurements took place before the samples were dried as described above. From these measurements, the relation of VOL to DW (VOL∝DW) could be calculated. The VOL and DW measurements were also used to calculate the wood density (ρ) of both species. The wood density calculated here is the average wood density of stem and crown samples combined. The estimates for ρ as DW per unit VOL of wood (g cm−3) originate from stem and crown disk samples, which were 3–5-cm thick slices of both species. Two densities were distinguished: ρexc excluding bark (as classical ρ) and ρinc including bark. For measuring ρexc, the bark was stripped off before the VOL and DW were accessed. Eq. (2) was used to calculate ρ
where ρ is the wood density in g/cm3, VOL is the fresh volume in cm3 before drying, and DW is the dry weight in g after drying at 105 °C. All ρ values are expressed on the basis of 105 °C dry weights with and without bark. The reason to additionally calculate ρ from VOL∝DW and compare our ρ with other densities found in literature was to check whether ρ alone could serve as explanation for the suspected differing AGB of the woody parts between North America and Europe.
To compare the performance of the two models (FW∝DW, VOL∝DW), DW was calculated for the sections of the trees which were weighed and additionally recorded volumetrically in the field (n Prunus = 32, n Robinia = 16) using each model separately, ideally both resulting in the same estimates for DW.
Combining the DW estimations with one another and applying them to the field measurements allowed calculating the total DW of each sample tree (Brown 1997).
Data analysis and modeling
The biomass functions presented in this paper are for above-ground parts of the trees, particularly for the total above-ground biomass (AGB), the biomass of stem (AGBstem), the biomass of crown (AGBcrown), and the biomass of the leaves (AGBleaf) as DW (105 °C until constant weight, cp. 2.3).
We tested eleven models (M01–M11) to estimate the biomass. In these models, BM represents the total dry weight of the biomass. Depending on the model, the predictor variables for the total weight of biomass were D as dbh or d_0.1, H as total height of trees, Crl as crown length, and Stl as stem length with Crl + Stl = H. The lower case letters (a–g) in the models are the estimated model parameters or scaling coefficients. The specific wood density ρ was not included into the models since no mixed species regressions (e.g., Brown et al. 1989; Chave et al. 2005; Dawkins 1961; Djomo et al. 2010) were calculated.
The vast majority of the biomass equations reviewed by Zianis et al. (2005) for Europe or reported by others (Feller 1992; Kaitaniemi 2004; Niklas 1994; Pilli et al. 2006; Ter-Mikaelian and Korzukhin 1997) took the form of Snell’s (1892) power equation BM = aD b. Additionally to the power equation (model 04), we added other models also only using D as predictor for biomass or using D in combination with other predictors (H, Crl, Stl) as reviewed by Ammer et al. (2004) and analyzed the effect on the predictive quality of the models. The same models were used to predict AGB, AGBstem, AGBcrown, and AGBleaf.
In order to correct for heterogeneous variation of the regression, a logarithmic transformation was applied (Wang 2006) for the predicted total weight (BM) and the predictor variables (e.g., D, H) resulting in, for example, ln(BM) = ln(a) + b ln(D) (see M04–M11). This is the standard method to estimate the scaling coefficients (a–g) through the least-squares regression of log-transformed data to account for the heteroscedasticity of the data (Djomo et al. 2010).
A correction factor was calculated to correct the systematic bias on the final biomass estimation of all equations due to the logarithmic transformation. A simple, first-order correction is calculated as (Finney 1941; Madgwick and Satoo 1975; Parresol 1999; Sprugel 1983):
The predicted biomass is multiplied by this factor to correct for the expected underestimation of the real values.To test and select the best statistical model, different indicators for the goodness-of-fit were used. The Akaike information criterion (AIC) was applied (Burnham and Anderson 2002; Johnson and Omland 2004). It is calculated as:
where l is the likelihood of the fitted model and EV is the total number of model parameters (explanatory variables) or regressors. Secondly, the residual standard error of estimation (RSE) is reported:
where Var (X) is the residual variance around the regression equation and DF are the degrees of freedom calculated as n–p, with n = number of observations and p = number of model parameters. Here, x represents the measurements and \( \overline{x} \) is the arithmetic mean of x.
The adjusted R 2adj as modification of R 2 is the third statistic reported, being based on the overall variance and the error variance (Crawley 2007).
with R 2 being the coefficient of determination, EV the total number of explanatory variables in the model, and n the sample size. Finally, the largest, the smallest, and the mean relative error δ(W) were calculated as suggested by Meyer (1938) and Schlaegel (1982):
with BMpred being the DW of biomass, predicted by the model and BM, the DW of biomass based on the field measurements.
Other statistics to evaluate the goodness-of-fit have been mentioned in literature (Parresol 1999; Schlaegel 1982). However, we believe the report provided enough information on the quality of the statistical models (Chave et al. 2005; Djomo et al. 2010).
The following eleven models (M01)–(M11) were used to estimate the different tree compartments AGB, AGBstem, AGBcrown, and AGBleaf, whereas the parameters of model 03, 09, 10, and 11 were reduced until all remaining explanatory variables had a significant impact on the model performance. These types of models have been commonly used, for example, by Brown et al. (1989), Chave et al. (2005), and Parresol (1999).
The linear models were:
-
(M01) BM = a + bD
-
(M02) BM = a + b (D 2 H)
-
(M03) BM = a + bD + cD 2 + dD 3
As transformed nonlinear models (nlm) we used:
-
(M04) ln(BM) = a + b ln(D) ↔ BM = exp(a)*D b
-
(M05) ln(BM) = a + b ln(D 2 H) ↔ BM = exp(a)*(D 2)b*H b
-
(M06) ln(BM) = a + b ln(D 2 H Crl) ↔ BM = exp(a) * (D 2)b * H b * Crlb
-
(M07) ln(BM) = a + b ln(D 2 H Stl) ↔ BM = exp(a) * (D 2)b * H b * Stlb
-
(M08) ln(BM) = a + b ln(D 2 H Crl Stl) ↔ BM = exp(a) * (D 2)b * H b * Crlb * Stlb
-
(M09) ln(BM) = a + b ln(D) + c ln(H) + d ln(Crl) + e ln(Stl) ↔ BM = exp(a)*(D)b*Hc*Crld *Stle
-
(M10) ln(BM) = a + b ln(D) + c ln(D)2 + d ln(D)3 ↔ BM = D b exp(a+c ln^2(D)+d ln^3(D))
-
(M11) ln(BM) = a + b ln(D) + c ln(D)2 + d ln(D)3 + e ln(Crl) + f ln(Stl) +g ln(H) ↔ BM = D b Crle Stlf H g exp(a+c ln^2(D)+d ln3 (D))
Models 01–11 were divided into two groups, the ones only using D as explanatory variable which are M01, M03, M04, and M10 and the models using at least one additional explanatory variable aside of D.
All statistical analyses, fittings, graphs, and validation tests were processed using the free software environment R (R Development Core Team 2011). Data distribution was verified visually using quantile plots, box-whisker plots, and frequency plots. These were complemented by using classical test like the Shapiro–Wilk test as whether the data came from a normal distribution or not. All data compared came from normal distributions. For comparing the variances, we used Fisher’s F test or Levene test. If these were not significantly different, we used Student’s t test or the ANOVA (for n > 2) to compare the groups. If the variances were significantly different, we applied the Welch test (Rasch et al. 1978). All tests comparing groups were two-sided for Student’s t test.
Results
Fresh weight to dry weight relation (FW∝DW)
The fresh to dry weight relation (FW∝DW) for the samples was best described using a simple linear model for both species. The differences between individual trees of the groups with dbh ≥ 7 cm and dbh > 7 cm (p = 0.4785, p = 0.5833), sampling seasons April 2010 and February 2011 (p = 0.1029), and sample position (p = 0.0719) concerning the woody samples collected from crown or stem were not significant. For the woody parts of both species, the proportion of variation in DW that could be explained by FW was above 0.99 for the adjusted R 2 (Table 2). The fit for the leaves is lower. The a value can be considered as dry content of the wood and slightly exceeds 60 % for both species. The high similarity of the fitted a value for the woody parts shows the related ratio of wood to moisture content for both species.
Volume to dry weight relation (VOL∝DW)
A ratio of weight to volume gives the density of an object, here the wood density ρ. Since the volumetric field measurements were conducted on plant parts, which were not stripped of bark, we have distinguished two densities (ρinc and ρexc). The average ρexc of about 0.6867 g cm−3 for P. serotina is significantly smaller (p < 0.0001, t = 5.6546) compared to the value of about 0.7361 g cm−3 for R. pseudoacacia, with a range across the two species from 0.6080 to 0.7662 g cm−3 for P. serotina and 0.6971 to 0.7959 g cm−3 for R. pseudoacacia (Fig. 2).
The calculations of the wood density including the bark (ρinc) have resulted in an average ρinc of 0.6105 g cm−3 for P. serotina which is also significantly smaller (p < 0.0001, t = 8.2043) compared to the value of about 0.6870 g cm−3 for R. pseudoacacia with a range across the two species from 0.5201 to 0.6592 g cm−3 for P. serotina and 0.6442 to 0.7421 g cm−3 for R. pseudoacacia (Fig. 2). The model describing the relation of VOL∝DW is shown in Table 3.
Conversion of field measurements to dry weight
FW∝DW and VOL∝DW allowed converting the field measurements to DW. However, applying the functions to the field samples that were not only weighed in the field but also measured volumetrically (n Prunus = 32, n Robinia = 16) produced different biomass estimates for the same samples. The calculated DW values are overestimated when using the volume-based model VOL∝DW in comparison with the DW values calculated by FW∝DW. This was especially true for R. pseudoacacia. For P. serotina, the difference was not as pronouncing, but also relevant (Fig. 3). So applying the uncorrected equations from Table 3 to plant parts for which only volumetric measurements had been conducted (stumps and parts too heavy to weigh) would have led to a systematic overestimation of these parts for some of the sample trees (Fig. 3a).
a Compares the calculated dry weight (DW) using either the fresh weight (FW) models (Table 2) as circles or the volume (VOL) models (Table 3) as triangles for the field samples which were not only weighed in the field but also measured volumetrically (n Prunus = 32, n Robinia = 16). Especially for R. pseudoacacia the calculated values from the VOL model are higher than the ones using the FW model. b Shows calculated DW after using the corrected VOL model (Table 3) compared to the value calculated by the FW models. The individual error is much smaller here compared to (a)
To avoid this, we corrected VOL∝DW by adjusting it to FW∝DW (Fig. 3b). We assumed the dry weight estimate from the model FW ∝ DW to be more precise than the estimates from VOL∝DW. So, the DW estimated by FW ∝ DW was explained with a new linear regression in which not FW was used as explanatory variable but VOL. This adjusts the overestimation to the values estimated by FW ∝ DW. The equations resulting from this correction can be found in Table 3. As to be noticed in Table 2 and 3, the relationship of VOL to DW is different to the relationship of FW to DW. The fitted a value for both species shows that DW for P. serotina is higher with a given VOL, whereas DW is more or less the same for both species at a given FW.
For all conversions of VOL or FW field data to DW, the equations presented in Table 2 and the corrected equations from Table 3 were used. The a value can be considered as ρ and is slightly different from the average wood density (see “Volume to dry weight relation”) because of using different samples.
Biomass estimation and model evaluation
We used the models M01–M11 to estimate the biomass for both species. For P. serotina, the analyses were based on two samples. Sample 2 focused on trees with a dbh ≥ 7 cm, while sample 1 included also smaller trees with a dbh < 7 cm (Table 1). For sample 2, AGB, AGBstem, and AGBcrown were estimated. For sample 1, AGB and AGBleaf were estimated. The analyses for R. pseudoacacia were based on only one sample. The minimum dbh for R. pseudoacacia was 5 cm and AGB, AGBstem, and AGBcrown were estimated by the models. The model estimates are based on the AGB measurements conducted in the field resulting in DW values from under 1 kg up to almost 790 kg (Table 4) for the whole trees.
Using AIC, R 2adj , RSE, and also considering the relative error, we tried to find the best models for each tree component. The three best models from the group of linear models (lm) and transformed nonlinear models (nlm) are listed in Table 5. Both groups are separated since they are not directly comparable using AIC or RSE. For all models, the values for the coefficient of determination (R 2adj ) ranged from 0.669 to 0.995, with a mean value of 0.926 suggesting consistently high estimation accuracies. Considering all models, the highest estimation accuracies (R 2adj ≥ 0.99) were achieved for R. pseudoacacia when estimating total, stem but also crown biomass. The lowest values (0.67 ≤ R 2adj < 0.83) were found for the P. serotina estimations of crown and leaf biomass. Even the best values for these compartments were also quite low, being 0.911 for the crown and 0.914 for the leaves. The nlm have mostly reached higher values than the linear models, except for AGBcrown and AGBtotal estimates of R. pseudoacacia and AGBtotal of P. serotina, whereas the difference is very small and can only be seen in the third decimal place behind the comma. In all other cases, the nlm reached higher values for R 2adj . From the nlm models, only depending on one explanatory variable M04 was most precise. For the models with more than one explanatory variable model types, M09 rank most frequently among the best three followed by M05. M07 and M08 were the least successful nlm models. Also models based on only one or two explanatory variables ranked among the best three eighteen times, whereas models with three or four explanatory variables can only be found in the ranking six times. Looking at the lm only using D as explanatory variable shows that the polynomial model types (M03) were more successful than the not squared model M01. M02 ranks on first position four times, M03 only three times.
Since models of the type of ln(y) = a + b ln(x) with x as dbh or dbh2*H are commonly used for the estimation of tree biomass, we were able to compare our estimates from M04 and M05 with those found by other authors (Brenneman et al. 1978; Hitchcock 1978; Wharton and Griffith 1993, 1998; Wiant et al. 1977). Those studies were conducted in the east of North America (Appalachian region: Virginia, Main, Tennessee). In all cases, our models estimate lower values for the AGB (Fig. 4).
a Shows our biomass equation (model 04) for P. serotina (dbh ≥ 7 cm, n = 47) in comparison with our equation for R. pseudoacacia and equations presented by other authors (Wiant et al. 1977, Brenneman et al. 1978, Wharton and Griffith 1993, 1998). b Shows our biomass equation for the regeneration (model 05) of P. serotina (n = 35) in comparison with the equation of Hitchcock (1978)
Discussion
Methodological considerations concerning biomass quantification and model evaluation
Above-ground biomass for individual trees or whole forest stands is often estimated as regression equation (Correia et al. 2010; Jenkins et al. 2003; Wharton and Griffith 1998) of certain explanatory variables (e.g., D, H, ρ). In this case, it is necessary to previously convert field measurements to DW. Usually, these field measurements consist of volumetric (Brown and Lugo 1984; Correia et al. 2010) or FW measurements (Djomo et al. 2010; Verwijst and Telenius 1999). FW is converted to DW by simply drying whole trees or samples of trees. The relation of both measurements is then used to convert FW measurements (cp. Table 2).
VOL is converted by making use of a function to convert VOL (cp. Table 3) or simply by using the wood density equation (Eq. 2) and solving it for DW (\( {\text{DW}} = \rho *{\text{VOL}} \)). Often, VOL is only available for the stem and is then used in combination with biomass expansion factors to expand the estimates to the crown or whole stand. But also here, the stem biomass estimate is based on VOL and ρ again. Applying either conversion here led to a systematic overestimation of DW like in Fig. 3, even more when considering the classical ρ as density of wood without bark (ρexc) for conversion.
In our case, we have estimated the DW as linear equation of the type y = ax + b (Table 3) where the explanatory variable was VOL and the values a and b were the fitted parameters. The a value in the function here is equivalent to ρ. The linear equation was based on woody samples including bark for which VOL was measured with high precision in a water bath as described above (cp. 2.3). However, VOL estimates of field samples—to which the equation was applied to—are based on volumetric measurements for the truncated cone formula (Eq. 1). For volumetric estimations based on Eq. 1 and only using a ρ value, which does not include the bark as density reducing factor, the AGB will always be overestimated. The thicker the bark, the higher the overestimation because bark has a lower density compared to wood. For this reason, we tried to estimate a ρ value, which considered the bark as density reducer, but still overestimated the values for DW. The overestimation was more pronounced for R. pseudoacacia than for P. serotina. A possible explanation for the overestimation of DW could be shrinkage as a result of storage and transport before the samples arrived in the laboratory where VOL was measured. Since R. pseudoacacia has a higher wood density than P. serotina (Fig. 2), shrinkage should be more pronounced in latter (Sachsse 1984). The thicker bark of R. pseudoacacia might diminish this assumption again slightly. However, here the overestimation was much higher for R. pseudoacacia, which is the opposite from what would have been expected if the overestimation were only a result of shrinkage. A likely explanation for this is the bark surrounding the wood. The bark of R. pseudoacacia is especially rough, ruptured, and thick. For some of the smaller dbh classes considered here, this is not yet so much the case for P. serotina, which has a thinner and smoother bark. When the tree becomes older, the bark is also more fissured and becomes scaly but remains thin (Godfrey 1988; Hare 1965; Uchytil 1991) and is not ruptured. When measuring the bottom and top diameter over bark for a certain part of the tree using a calliper or girthing tape, the crevices and hollow parts of the bark are not taken into account. But while calculating the volume using the formula for the truncated cone, these parts of the bark are considered as to be filled in and the resulting total volume will be overestimated and must be corrected. For this reason, we decided to not use the ρinc estimates presented here (Fig. 2) but to correct the estimates by relating these to the biomass they should have had through conversion from FW to DW (Table 3). Comparing the mean ρinc for P. serotina (0.6105) and R. pseudoacacia (0.6870) with the uncorrected value from Table 3 shows that our estimates for ρinc are quite similar to VOL∝DW based on the exact water displacement measurements. But comparing it to the corrected value of parameter “a” for each species from Table 3 (0.5631 and 0.4967) shows to which degree the structure of the bark (subsequently called bark effect) would affect biomass estimations. For P. serotina, the bark effect only results in a difference of about 5 % (0.0474), whereas for R. pseudoacacia the difference would be almost 20 % (0.1903). The bark effect will be more pronounced for species that tend to develop a ruptured and thick bark. The effect will be stronger for thinner dbh classes with a higher proportion of bark compared to thicker dbh classes.
Therefore, when using classical wood densities that do not include the bark, we recommend revising them downwards. Also, we should not disregard that wood densities tend to vary not only with tree species, but also growth conditions and parts of the trees measured, where the main stem often has a higher ρ compared to smaller branches of the crown (here about 0.71 vs. 0.66 for P. serotina and 0.75 vs. 0.71 for R. pseudoacacia). Additionally, growth speed has an influence on ρ (Bouriaud et al. 2005). Trees growing slower generally produce wood with higher density when compared to trees of the same species, which have grown faster (Zhang 1995; Zobel and van Buijtenen 1989). All this recommends using wood densities carefully when used for biomass estimation.
For species, for which the bark effect could be more relevant, we recommend to try to not only estimate a ρ which includes the bark, but also conduct volumetric measurements on plant parts that can be weighed in field to adjust the density estimates. As shown here, even the estimates including the bark were too high (Fig. 3a). Of course, this bears the risk that these measurements are mainly conducted on thinner parts of the tree, which again in turn would overvalue the bark effect for the reason mentioned above.
Predictive power of the models and model evaluation
The results have shown that models based on one or two explanatory variables perform quite well in comparison with models based on three or four explanatory variables. However, for the models using an additional explanatory variable aside of D, their performance depended mainly on what was being estimated. Noticeably, when AGBcrown or AGBstem were estimated, the nlm models performing best were the ones including the length of the tree compartment being estimated. Here, this means the introduction of Stl and Crl. Adding H as second explanatory variable has improved the models in some cases, but not as much as might have been expected. For instance, when estimating the total AGB, the models including H ranked higher than the models only including D. However, the higher ranks were only based on very small differences in R 2adj . As also found by Röhle (2009), Röhle et al. (2006) and Djomo et al. (2010) including only H of the trees did not improve the estimation of the models considerably. And in the case of AGBtotal of young P. serotina, not only H but also Crl was necessary to improve the model. Crl seemed to play a greater role for the biomass of very small plants. The additional cost of measuring H in the field in relation to the improvement it brings for the estimation can be questioned since only using D (dbh or d_0.1) led to comparably good results. On the other side, the good performance of model type M09, but also of some of the other models, suggests adding Crl or Stl to the height measurements if these are conducted anyhow, since the measurement of one additional height is negligible extra work but improves the models’ accuracy. When using D as only explanatory variable, we suggest using M04 for biomass estimations (Table 6) or a model similar to M10 which can always turn into M04 due to simplification based on significance of the explanatory variables. From these models, M04 has performed best as also found by Parresol (1999) and Djomo et al. (2010). When using more than one explanatory variable, models of the type M09 or model M05 performed well. M09 has to be simplified until all remaining explanatory variables have a significant impact on the models estimation accuracy.
Also M04 in combination with the equation type M05 can often be found in literature (Ter-Mikaelian and Korzuklin 1997; Zianis et al. 2005) and allows comparing own data with work of others. Comparing a list of 279 allometric biomass equations Zianis and Mencuccini (2004) found a value close to 2.36 for parameter b describing the proportionality between the relative increment of biomass and the diameter. According to Djomo et al. (2010), a value of about 2.67 is predicted from fractal models by West et al. (1997, 1999), Brown and West (2000), Enquist (2002) and Niklas (1994, 2004). The b value ranged from 2.01 to 2.93 (Table 6) with an average of 2.44 in this study. It is said that power equations as equation type (Pilli et al. 2006; Zianis and Mencuccini 2004), for analyses of forest biomass perform so well because growing plants maintain the weight proportion between different woody parts (Djomo et al. 2010; West et al. 1997, 1999). Here, power functions have also performed quite well, even though weight proportion between different woody parts as defined for this study (cp. 2.2) was not maintained. For small dbh, AGBcrown is smaller by a factor of up to almost seven. With increasing dbh, AGBcrown grew closer to AGBstem. The variance of the weight proportions was quite great (Fig. 5).
Not only with regard to the AGB but also when estimating the plant components AGBstem and AGBcrown, R. pseudoacacia was better described in comparison with P. serotina. This seems to show that all tree components of R. pseudoacacia could be estimated quite well.
Among each species separately, the estimations for AGB and AGBstem were more precise compared to AGBcrown or AGBleaf, whereas the last mentioned was most hard to predict. The trouble in predicting AGBcrown could be related to the crown definition used in this work. When defining the beginning of a crown as point where a straight trunk axis could not be recognized any longer, we took into account that this could be the case after only a couple of meters, for example, if the tree had forks. However, we could not have set a diameter limit to distinguish stem and crown and as explained topping off was not possible either in most cases.
The fact that AGBleaf is hard to estimate was expected. Wang (2006) also found leaves most hard to describe. His model resulted in R 2 = 0.818. Our models averaged in R 2adj = 0.866 (0.744–0.918). Leaf or needle biomass might be stronger affected by other stand characteristics like social status in the stand according to Kraft (1884) or stand density (Barclay et al. 1986; Keane and Weetman 1987; Satoo and Madgwick 1982). Also leaves are more susceptible to weather conditions. For a defined amount of leaves, FW measurements can vary quite a lot when dry, moist (e.g., from morning drew) or wet (e.g., from rain), while DW measurements will be the same in each case. While the leaf samples were collected in April 2010, it rained on a couple of days. Also the moisture on the leaves of the trees sampled was higher during the morning in comparison with the afternoon. Even though we tried to collect representative samples, this might have increased the error for the leaf dataset. For this reason, we believe that the phenology of the leaves should have played a minor role as explanation for the increased error and weaker estimates in this case.
While comparing the model performance for P. serotina with R. pseudoacacia, it should be considered that estimates for R. pseudoacacia were based on a smaller sample (13) than P. serotina (47). Also the diameter and height range were smaller for R. pseudoacacia (5–24.3 cm, 7.4–16.4 m) compared to latter (7–35.9 cm, 5.9–17.5 m) with a variance of 37.87 and 43.31 for the dbh and 9.68 and 7.13 for tree height, respectively. And finally also the weight dimensions had a variance of 4,873.17 (5.11–228.8 kg) and 18,680.3 (9.85–786.3 kg) for R. pseudoacacia and P. serotina, so the variance was about four times as high for P. serotina which could also serve as explanation why the model fit for R. pseudocacacia was higher. Nevertheless, according to the empirical rules by Garson (2011), the sample size was appropriate for the regression analysis presented here and is still among the most usual amount of sampled trees in other studies conducted in Europe (Zianis et al. 2005). Also the variances of the descriptive parameters for each sample tree did not indicate great differences among the two samples. Consequently, it might be possible that growth in relation to weight proportion between different plant parts is more homogeneous in R. pseudoacacia than in P. serotina.
Comparing the biomass functions for southern Europe to North America
The temperate sub-continental climate conditions in the study area are more comparable to Central Europe than to the Mediterranean. These conditions are similar to the conditions of some other Central European regions in Western Germany, Northern France, and the Netherlands where P. serotina also occurs even though precipitation and temperature might be slightly lower there on average. Considering this, the biomass functions of this study can be applied to sites with similar conditions. However, further biomass functions for Europe are desirable.
P. serotina is native to eastern North America and grows from eastern Texas north to western Minnesota, and eastward to the Atlantic from central Florida to Nova Scotia (Little 1979; Uchytil 1991), with some outlying populations and varieties showing other distributions. We have compared our biomass functions with others developed in the Appalachians and the West Virginia hardwoods and have found that our models estimate lower values for biomass (Fig. 4). The difference to Wiant’s model (Wiant et al. 1977) is small but Brenneman’s (Brenneman et al. 1978) and finally Wharton’s model (Wharton and Griffith 1993, 1998) clearly estimate higher values. Additionally, the model for young P. serotina of Hitchcock (1978) also estimates much higher values than our model. Wood densities were estimated to be able to exclude ρ as explanation for lower biomass estimations. The ρexc measurements provide the base for this by comparing our measurements with the values given by other authors. Since many of the values available come from the industry, often only ρ at about 15 % moisture content is reported and not the DW since the first mentioned is more relevant to the industry. So here, the estimated wood densities excluding bark are comparable to other studies. For R. pseudoacacia, Knigge and Schulz (1966) present a value of 0.73 g cm−3 ranging from 0.54 to 0.87 g cm−3. Nennewitz et al. (2005) and Sell (1997) reported values of 0.73 g cm−3 for R. pseudoacacia (here 0.7361 g cm−3) and 0.6–0.63 g cm−3 for P. serotina (here 0.6867 g cm−3) for samples with a wood moisture content of 15 %, so the value for the DW would be slightly lower, like the density of 0.54 g cm−3 reported by Corkhill (1989). Jenkins et al. (2003) reported a wood-specific gravity of only 0.47. Their work is based on Williams and McClenahen (1984) who estimated the biomass for seedlings, sprouts, and saplings which might explain the lower ρexc. So our ρexc even seems to be slightly higher and cannot be used as explanation for lower biomass values.
The site conditions could be an explanation, suggesting greater tree height at a comparable diameter in the native range of P. serotina. In our study area, trees from the upper layer have heights ranging between 20 and 25 m to a maximum of around 30 m. The P. serotina and R. pseudoacacia trees in this study were all <20 m. In the east of North America, P. serotina can reach 38 m (Duncan and Duncan 1988) but southwestern varieties are typically much smaller (Uchytil 1991).
P. serotina grows on a variety of soils in North America (Marquis 1990) and develops well, except on the very wettest and very driest soils (Hough 1965). For Europe, Wendorff (1952) found P. serotina to grow better on soils containing more clay than soils containing more sand. In this respect, soil conditions of mesic woods as found in the study area should hence not affect productivity negatively. In its region of origin, P. serotina grows with an average annual precipitation of 970–1,120 mm (Kowarik 2010). On the study site, mean annual precipitation amounts to about 850–980 mm (Castelnuovo and Tonetti 2003; Ferré et al. 2005) so the sum of precipitation could be slightly too low. According to Marquis (1990), summer growth conditions seem to be more important than annual averages, meaning the distribution of the precipitation and temperature throughout the year. In North America, P. serotina grows well under conditions that are cool (min, 11–16 °C; max, 27–29 °C) and moist (510–610 mm) during the summer (Marquis 1990). The growing season lasted from end of March to end of September in the study area for the years from 1993 to 2004 (Castelnuovo and Tonetti 2003). During this time, temperatures ranged from 12 to 24 °C per month on average with extreme temperatures close to 0 °C and 40 °C. Average precipitation during the growing season ranged from 75 to 665 mm. So, the study area does not seem to be as moist as preferred by P. serotina during the growing season which could reduce the productivity of the plants. Interspecific competition can also affect growth increment (Mölder et al. 2011) but seems quite unlikely in this context because it would mean that European competitors of P. serotina suppress its growth more than in its native range of distribution. In this case, P. serotina would not be expected to be as competitive as it is. Even under very good growth conditions, also other species from North America do not show comparable growth performances in Europe (e.g., Pseudotsuga menziesii, R. pseudoacacia) when compared to their region of origin (Roloff et al. 1994).
While comparing our results with other authors, one should keep in mind that we adjusted the models to our data, which means that we had to extrapolate the other models beyond the original range of data in some cases, which is always fraught with difficulties (Crawley 2007). For example, Hitchcock (1978) estimated his values for plants with a diameter of 1.4–4 cm and a height of 0.3–6.4 m. Our measurements are slightly higher (Table 1). The trees measured by Wiant et al. (1977) had a diameter of 12.7–40.6 cm (i.e., 5–16 in.), whereas we start our measurements at 7 cm. We could not find information on sample size. Brenneman et al. (1978) only stated their trees sampled were ≥12.7 cm and that they had 26 trees. Wharton and Griffith (1993, 1998) did not present the diameter range sampled and only gave the median stem diameter for some species presented, but not for P. serotina. They did not mention the sample size either. Also, their model includes the biomass of the leaves, whereas the other models only estimate the woody biomass. However, they give the foliage biomass as percentage of AGB and it ranged from 3.48 to 1.75 % depending on the diameter (poletimber to large sawtimber).
Conclusions
Our study shows that P. serotina, like other species introduced from North America, is less productive in Europe when compared to North America, due to smaller achieved growth heights. Soil conditions have a strong influence on maximum heights. However, here too low moisture levels during the growing season might also be the explanation for the reduced biomass production.
When estimating the biomass of P. serotina and also R. pseudoacacia with biomass functions, dbh as only explanatory variable leads to good results and might be sufficient for calculations, especially for individuals originating from the same site. However, models including total height, crown length or stem length as explanatory variables perform better. For this reason, we recommend to not only record the height of the trees but to add stem or crown length measurements if further measurements aside of the dbh are being conducted to estimate the biomass. When estimating the biomass from volumetric measurements in combination with the truncated cone formula, we recommend correcting the estimates downwards, especially for tree species with ruptured bark. Furthermore, when using these volumetric measurements with wood density to estimate the biomass, we recommend considering the bark of the trees as density reducing factor since volumetric measurements include the bark and classical density measurements are based on samples excluding the bark. Whenever possible, adding fresh weight measurements to the volumetric measurements allows comparing the estimates for the dry weight. The biomass functions presented here can be used in combination with most forest inventory data and are the basis for area-related biomass estimates of the two species.
Abbreviations
- DW:
-
Absolute dry weight (samples dried to a constant weight)
- FW:
-
Fresh weight measured in the field
- VOL:
-
Volume
- VOL∝DW:
-
Volume to dry weight relation
- FW∝DW:
-
Fresh weight to dry weight relation
- Ρ:
-
Wood density
- ρinc :
-
Wood density with bark
- ρexc :
-
Wood density without bark
- AGB:
-
Total above-ground biomass (with/without leaves dep. on sample)
- AGBstem :
-
Above-ground stem biomass
- AGBcrown :
-
Above-ground crown wood biomass (excluding the leaves)
- AGBleaf :
-
Above-ground leaf biomass
- lm:
-
Linear models
- nlm:
-
Transformed nonlinear models
References
Ammer C, Brang P, Knoke T, Wagner S (2004) Methoden zur waldbaulichen Untersuchung von Jungwüchsen. Forstarchiv 75:83–110
Barclay HJ, Pang PC, Pollard DFW (1986) Aboveground biomass distribution within trees and stands in thinned and fertilized Douglas-fir. Can J For Res 16:438–442
Bouriaud O, Leban J-M, Bert D, Deleuze C (2005) Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol 25(6):651–660
Brehm K (2004) Erfahrungen mit der Bekämpfung der Spätblühenden Traubenkirsche Prunus serotina in Schleswig-Holstein. In: Schriftenreihe LANU SH—Natur 10, Neophyten in Schleswig-Holstein: Problem oder Bereicherung? Landesamt für Natur und Umwelt des Landes Schleswig-Holstein, pp 66–78
Brenneman B, Frederick DJ, Gardner WE, Schoenhofen LH, Marsh PL (1978) Biomass of species and stands of West Virginia hardwoods. In: Proceedings, central hardwood forest conference II; 14–16 November, West Lafayette, pp 159–178
Brosemann GA, Krug W (2006) Erfahrungen mit der Spätblühenden Traubenkirsche. AFZ- Der Wald 5:243–246
Brown S (1997) Estimating biomass and biomass change of tropical forests: a primer. FAO forestry paper 134. Rome, Italy
Brown S, Lugo AE (1984) Biomass of tropical forests: a new estimate based on forest volumes. Science 223:1290–1293
Brown RL, Peet RK (2003) Diversity and invasibility of southern Appalachian plant communities. Ecology 84:32–39
Brown JH, West GB (2000) Scaling in biology. Oxford University Press, New York
Brown S, Gillespie AJR, Lugo AE (1989) Biomass estimation methods for tropical forests with applications to forest inventory data. Forest Sci 35(4):881–902
Burnham KP, Anderson DR (2002) Model selection and inference. A practical information-theoretic approach, 2nd edn. Springer, Berlin
Burwell CC (1978) Solar biomass energy: an overview of U.S. potential. Science 199:1041–1048
Castelnuovo M, Tonetti R (2003) Report: Indagini diagnostiche sul deperimento della farnia nei boschi della Valle del Ticino
Chabrerie O, Verheyen K, Saguez R, Decocq G (2008) Disentangling relationships between habitat conditions, disturbance history, plant diversity, and American black cherry (Prunus serotina Ehrh.) invasion in a European temperate forest. Divers Distrib 14(2):204–212
Chapin FS III, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Díaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Chapman AG (1935) The effect of black locust on associated species with special reference to forest trees. Ecol Monogr 5:37–60
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Fölster H, Fromard F, Higuchi N, Kira T, Lescure J-P, Nelson BW, Ogawa H, Puig H, Riéra B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145(1):87–99
Closset-Kopp D, Chabrerie O, Valentin B, Delachapelle H, Decocq G (2007) When Oskar meets Alice: does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? Forest Ecol Manag 247:120–130
Corkhill T (1989) The complete dictionary of wood. Dorset Pr, New York
Correia AC, Tomé M, Pacheco CA, Faias S, Dias AC, Freire J, Carvalho PO, Pereira JS (2010) Biomass allometry and carbon factors for a mediterranean pine (Pinus pinea L.) in Portugal. Forest Syst 19(3):418–433
Crawley MJ (2007) The R book. Wiley, Chippenham
Cronk QCB, Fuller J (1995) Plant invaders: the threat to natural ecosystems. World wide fund for nature. Chapman & Hall, London
Cuddington K, Hastings A (2004) Invasive engineers. Ecol Model 178:335–347
Dawkins HC (1961) Estimating total volume of some Caribbean trees. Caribb For 22:62–63
Djomo AN, Ibrahima A, Saborowski J, Gravenhorst G (2010) Allometric equations for biomass estimations in Cameroon and pan moist tropical equations including biomass data from Africa. Forest Ecol Manag 260(10):1873–1885
Duncan WH, Duncan MB (1988) Trees of the southeastern United States. The University of Georgia Press, Athens
Enquist BJ (2002) Universal scaling in tree and vascular plant allometry: toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol 22:1045–1064
Feller MC (1992) Generalized versus site-specific biomass regression equations for Pseudotsuga menziesii var. menziesii and Thuja plicata in coastal British Columbia. Bioresour Technol 39:9–16
Ferré C, Leip A, Matteucci G, Previtali F, Seufert G (2005) Impact of 40 years poplar cultivation on soil carbon stocks and greenhouse gas fluxes. Biogeosciences 2:897–931
Finney DJ (1941) On the distribution of a variate whose logarithm is normally distributed. J R Stat Soc Ser 7(2):155–161
Garson GD (2011) Sample size, from statnotes: topics in multivariate analysis. Retrieved 01/04/2011 from http://faculty.chass.ncsu.edu/garson/PA765/statnote.htm
Godfrey RK (1988) Trees, shrubs, and woody vines of northern Florida and adjacent Georgia and Alabama. The University of Georgia Press, Athens
Göhre K (1952) Die Robinie und ihr Holz. Deutscher Bauernverlag, Berlin
Hall DO, Mynick HE, Williams RH (1991) Cooling the greenhouse with bioenergy. Nature 353:11–12
Hare RC (1965) Contribution of bark to fire resistance of southern trees. J Forest 63(4):248–251
Higgins SI, Richardson DM, Crowling RM, Trinder-Smith TH (1999) Predicting the landscape-scale distribution of alien plants and their threat to plant diversity. Conserv Biol 13:303–313
Hitchcock HC (1978) Above ground tree weight equations for hardwood seedlings and saplings. TAPPI 61:119–120
Hough AF (1965) Black cherry (Prunus serotina Ehrh.). In: Fowells HA (ed) Silvics of forest trees of the United States. comp. U.S. Department of Agriculture, Agriculture Handbook 271. Washington, pp 539–545
Jenkins JC, Chojnacky DC, Heath LS, Birdsey RA (2003) National-scale biomass estimators for United States tree species. Forest Sci 49(1):12–35
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Jurko A (1963) Die Veränderung der ursprünglichen Waldvegetation durch die Introduktion der Robinie (in Czech, German summary) Ceskoslovensá ochrana pribrody 1, 56–75
Kaitaniemi P (2004) Testing allometric scaling laws. J Theor Biol 228:149–153
Keane MG, Weetman GF (1987) Leaf area sapwood cross-sectional area relationships in repressed stands of lodgepole pine. Can J For Res 17:205–209
Knigge W, Schulz H (1966) Grundriss der Forstbenutzung: Entstehung, Eigenschaften. Verwertung und Verwendung des Holzes und anderer Forstprodukte, Parey
Kowarik I (2010) Biologische invasionen: Neophyten und Neozoen in Mitteleuropa, 2nd edn. Ulmer, Stuttgart
Kraft G (1884) Beiträge zur Lehre von den Durchforstungen, Schlagstellungen und Lichtungshieben. Klindworth’s Verlag, Hannover
Little EL Jr (1979) Checklist of United States trees (native and naturalized). Forest Service Agric Handb. 541. U.S. Department of Agriculture, Washington
MA (2005) Millennium Ecosystem Assessment, 2005. Ecosystems and human well-being: synthesis. Island Press, Washington
Madgwick HAI, Satoo T (1975) On estimating the above ground weights of tree stands. Ecology 56:1446–1450
Marquis DA (1990) Prunus serotina Ehrh. Black cherry, In: Burns RM, Honkala BH (eds) Silvics of North America, vol 2. Hardwoods. U.S. Department of Agriculture, Forest service: Agric Handb. 654. Washington, pp 594–604
Meyer HA (1938) The standard error of estimate of tree volume from logarithmic volume equation. J For 36:340–342
Minelli A, Ruffo S, Stoch F (2002) Woodlands of the Po Plain, Italian Habitats 3. Ministero dell’Ambiente e della Tutela del Territorio Museo Friulano di Storia Naturale, Undine
Mölder I, Leuschner C, Leuschner HH (2011) d13C signature of tree rings and radial increment of Fagus sylvatica trees as dependent on tree neighborhood and climate. Trees 25:215–229
Mooney HA (1999) Species without frontiers. Nature 397:665–666
Nennewitz I, Nutsch W, Peschel P, Seifert G (2005) Tabellenbuch Holztechnik, 4th edn. Europa Lehrmittel Verlag, Haan
Nentwig W, Bacher S, Cock M, Dietz HJ, Gigon A, Wittenberg R (2005) Biological invasions—from ecology to control. Neobiota 6, Berlin
Niklas KJ (1994) Plant allometry. The Scaling of Form and Process. The University of Chicago Press, Chicago
Niklas KJ (2004) Plant allometry: is there a grand unifying theorem? Biol Rev 79:871–889
Ohlrogge J, Allen D, Berguson B, DellaPenna D, Shachar-Hill Y, Stymne S (2009) Driving on biomass. Science 324:1019–1020
Ortega YK, Pearson DE (2005) Weak versus strong invaders of natural plant communities: assessing invasibility and impact. Ecol Appl 15:651–661
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Inv 1:3–19
Parresol BR (1999) Assessing tree and stand biomass: a review with examples and critical comparisons. For Sci 45:573–593
Pilli R, Anfodillo T, Carrer M (2006) Towards a functional and simplified allometry for estimating forest biomass. Forest Ecol Manag 237:583–593
R Development Core Team (2011) R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing. http://www.R-project.org
Rasch D, Herrendörfer G, Bock J, Busch K (1978) Verfahrensbibliothek, Bd. 1 and 2, Deutscher Landwirtschaftsverlag, Hannover
Richardson DM, PyÍek P, Rejmánek M, Barbour MG, Panetta FD, West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107
Röhle H (2009) Arbeitskreis Biomasse: Verfahrenseempfehlungen zur Methodik der Biomasseermittlung in Kurzumtriebsbeständen. DVFFA—Sektion Ertragskunde, Jahrestagung, 220–226
Röhle H, Hartmann K-U, Gerold D, Steinke C, Schröder J (2006) Aufstellung von Biomassefunktionen für Kurzumtriebsbestände. Allg Forst u J-Ztg 177:178–187
Roloff A, Weisgerber H, Lang U, Stimm B, Schütt B (1994) Enzyklopädie der Holzgewächse. Handbuch und Atlas der Dendrologie. Wiley-VCH, Weinheim
Rostrup-Nielsen JR (2005) Making fuels from biomass. Science 308:1421–1422
Sachsse H (1984) Einheimische Nutzhölzer und ihre Bestimmung nach makroskopischen Merkmalen. Pareys Studientexte Nr. 44, Hamburg, Berlin
Satoo T, Madgwick HAI (1982) Forest biomass. Kluwer, Dordrecht
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18:561–566
Schlaegel BE (1982) Testing, reporting, and using biomass estimation models. In: Gresham CA, Belle W (eds) Proc. of the 3rd annual southern forest biomass workshop. Clemson Univ, Clemson, SC 137, pp 95–112
Sell J (1997) Eigenschaften und Kenngrössen von Holzarten. Arbeitsgemeinschaft für d. Holz. fourth ed. Baufachverlag, Lignum
Snell O (1892) Die Abhängigkeit des Hirngewichts von dem Körpergewicht und den geistigen Fähigkeiten. Arch Psychiatr 23:436–446
Solbrig OT (1991) Ecosystems and global environmental change. In: Corell RW, Anderson PA (eds) Global environmental change. Springer, Berlin, pp 97–108
Sprugel DG (1983) Correcting for bias in log-transformed allometric equations. Ecology 64:209–210
Starfinger U (1991) Population biology of an invading tree species Prunus serotina. In: Seitz A, Loeschke V (eds) Species conservation: a population biology approach. Birkhäuser Verlag, Basel, pp 171–184
Tabacchi G, Di Cosmo L, Gasparini P (2011) Aboveground tree volume and phytomass prediction equations for forest species in Italy. Eur J Forest Res. doi:10.1007/s10342-011-0481-9. (Published online: 12 February 2011)
Ter-Mikaelian MT, Korzukhin MD (1997) Biomass equations for sixty-five North American tree species. Forest Ecol Manag 97(1):1–24
Uchytil RJ (1991) Prunus serotina. In: Fire effects information system, online. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). http://www.fs.fed.us/database/feis/ (05/2011)
UNESCO (2005) MAB Biosphere Reserves Directory. Biosphere Reserve Information, Italy Valle del Ticino, online http://www.unesco.org/mabdb (04/2011)
Verwijst T, Telenius B (1999) Biomass estimation procedures in short rotation forestry. Forest Ecol Manag 121(1–2):137–146
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84:218–228
Wang C (2006) Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. Forest Ecol Manag 222(1–3):9–16
Wein K (1930) Die erste Einführung nordamerikanischer Gehölze in Europa. Teil I. Mitt Dtsch Dendrol Ges 42:137–163
Wein K (1931) Die erste Einführung nordamerikanischer Gehölze in Europa. Teil II. Mitt Dtsch Dendrol Ges 43:95–154
Wendorff v G (1952) Die Prunus serotina in Mitteleuropa. Eine Waldbauliche Monographie. Diss an der mathematisch naturwissenschaftlichen Fakultät der Univ. of Hamburg
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometry scaling laws in biology. Science 276:122–126
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400:664–667
Wharton EH, Griffith DM (1993) Methods to estimate total forest biomass for extensive forest inventories: applications in the northeastern U.S. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station
Wharton E, Griffith DM (1998) Estimating total forest biomass in Maine, 1995. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station
Wiant HV, Sheetz CE, DeMoss JC, Castaneda F (1977) Tables and procedures for estimating weights of some appalachian hardwoods. West Virginia University, Agricultural and Foresty Experiment station: Internet Archive. Bd. 36. W. Va. Agric. Exp. Stn. Bull. 659(T) http://www.archive.org/details/tablesprocedures659wian
Williams RA, McClenahen JR (1984) Biomass prediction equations for seedlings, sprouts, and saplings of ten central hardwood species. For Sci 30:523–527
Williamson M (1999) Invasions. Ecography 22:5–12
Zhang SY (1995) Effect of growth rate on wood specific gravity and selected mechanical properties in individual species from distinct wood categories. Wood Sci Technol 29(6):451–465
Zianis D, Mencuccini M (2004) On simplifying allometric analyses of forest biomass. Forest Ecol Manag 187:311–332
Zianis D, Muukkonen P, Mäkipää R, Mencuccini M (2005) Biomass and stem volume equations for tree species in Europe. Silva Fenn 4:1–63
Zobel BJ, Buijtenen JP (1989) Wood variation: its causes and control. Springer, Berlin
Acknowledgments
We would like to thank the Marianne and Dr. Fritz-Walter Fischer Foundation within the Stifterverband für die Deutsche Wissenschaft for funding our research and the DAAD (German Academic Exchange Service) VIGONI program for supporting project-based exchange. We acknowledge the anonymous reviewers of the manuscript for their constructive comments and recommendations. We also wish to acknowledge the assistance given to us by Dr. Fulvio Caronni from the Ticino Park management. He generously made facilities available for the study and let us profit from his long-time experience in research and fieldwork. Finally, we are especially grateful to Eike Feldmann and Karl-Heinz Heine who were a big help and assistance while collecting the data.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Berger.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Annighöfer, P., Mölder, I., Zerbe, S. et al. Biomass functions for the two alien tree species Prunus serotina Ehrh. and Robinia pseudoacacia L. in floodplain forests of Northern Italy. Eur J Forest Res 131, 1619–1635 (2012). https://doi.org/10.1007/s10342-012-0629-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-012-0629-2