Abstract
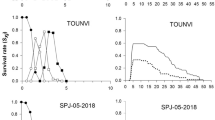
The tomato red spider mite, Tetranychus evansi, is an emerging pest of solanaceous crops. Two distinct genetic lineages (I and II) have been identified, lineage I having a much wider geographic distribution than lineage II. This has been attributed to differences in cold hardiness that make lineage I better adapted to colonize the coldest parts of the invaded area. However, other factors such as the ability to exploit different hosts may also be involved. In this work, we compared the performance of the Nice (lineage I) and Perpignan (lineage II) strains of T. evansi on two frequent host plants for this species: black nightshade, Solanum nigrum, and cultivated tomato, S. lycopersicum. In general, Nice strain mites performed better (higher fecundity, lower offspring mortality, bigger egg size and lower percentage of males) than Perpignan strain mites when both: (1) they were reared and tested on the same host plant (S. lycopersicum or S. nigrum); and (2) when shifted from S. nigrum to S. lycopersicum and vice versa. Digestive proteases showed also higher expression in Nice strain mites than in Perpignan strain mites, independently of their plant host, potentially reflecting a more efficient proteolytic digestion of plant proteins. However, no differences in detoxification enzyme (P450, esterases and glutathione S-transferases) activities were found when the two strains were compared. In conclusion, our results demonstrate that Nice strain mites exhibited life history traits leading to higher fitness on two different hosts, which may be related with the higher invasive potential and outbreak risks of mites from lineage I.



Similar content being viewed by others
References
Alba JM, Schimmel BCJ, Glas JJ, Ataide LM, Pappas ML, Villarroel CA, Schuurink RC, Sabelis MW, Kant MR (2015) Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol 205:828–840
Azandémè-Hounmalon GY, Fellous S, Kreiter S, Fiaboe KKM, Subramanian S, Kungu M, Martin T (2014) Dispersal behavior of Tetranychus evansi and T. urticae on tomato at several spatial scales and densities: implications for Integrated Pest Management. PLoS ONE 9:e95071
Baker HG (1965) Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species, Asilomar, California, USA, 1965. Academic, New York, pp 147–172
Boubou A, Migeon A, Roderick GK, Navajas M (2011) Recent emergence and worldwide spread of the red tomato spider mite, Tetranychus evansi: genetic variation and multiple cryptic invasions. Biol Invasions 13:81–92
Boubou A, Migeon A, Roderick GK, Auger P, Cornuet J-M, Magalhaes S, Navajas M (2012) Test of colonisation scenarios reveals complex invasion history of the red tomato spider mite Tetranychus evansi. PLoS ONE 7:e35601
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carrillo L, Martinez M, Ramessar K, Cambra I, Castañera P, Ortego F, Diaz I (2011) Expression of a barley cystatin gene in maize enhances resistance against phytophagous mites by altering their cysteine-proteases. Plant Cell Rep 30:101–112
EPPO (2004) European and Mediterranean Plant Protection Organization. Reporting Service no. 05–2004 Num. article: 2004/080. https://gd.eppo.int/reporting/article-1601
Falara V, Alba JM, Kant MR, Schuurink RC, Pichersky E (2014) Geranyllinalool synthases in Solanaceae and other angiosperms constitute an ancient branch of diterpene synthases involved in the synthesis of defensive compounds. Plant Physiol 166:428–441
Ferragut F, Garzón-Luque E, Pekas A (2013) The invasive spider mite Tetranychus evansi (Acari: Tetranychidae) alters community composition and host-plant use of native relatives. Exp Appl Acarol 60:321–341
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol 45:341–369
Gotoh T, Sugimoto N, Pallini A, Knapp M, Hernandez-Suarez E, Ferragut F, Ho CC, Migeon A, Navajas M, Nachman G (2010) Reproductive performance of seven strains of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) at five temperatures. Exp Appl Acarol 52:239–259
Grbic M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbic V, Osborne EJ, Dermauw W, Ngoc PCT, Ortego F, Hernández-Crespo P, Diaz I, Martinez M, Navajas M, Sucena E, Magalhaes S, Nagy L, Pace RM, Djuranovic S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, Hutter JL, Hudson SD, Velez M, Yi SV, Zeng J, Pires-daSilva A, Roch F, Cazaux M, Navarro M, Zhurov V, Acevedo G, Bjelica A, Fawcett JA, Bonnet E, Cindy Martens C, Baele G, Wissler L, Sanchez-Rodriguez A, Tirry L, Blais C, Demeestere K, Henz SR, Gregory TR, Mathieu J, Verdon L, Farinelli L, Schmutz J, Lindquist E, Feyereisen R, Van de Peer Y (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492
Gutierrez J, Etienne J (1986) Les Tetranychidae de l’ile de la Réunion et quelques-uns de leurs prédateurs. Agron Trop 41:84–91
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52:231–253
Macke E, Magalhães S, Khan HDT, Luciano A, Frantz A, Facon B, Olivieri I (2010) Sex allocation in haplodiploids is mediated by egg size: evidence in the spider mite Tetranychus urticae Koch. Proc R Soc Lond B 278:1054–1063
Meynard CN, Migeon A, Navajas M (2013) Uncertainties in predicting species distributions under climate change: a case study using Tetranychus evansi (Acari: Tetranychidae), a widespread agricultural pest. PLoS ONE 8:e66445
Migeon A, Dorkeld F (2006–2015) Spider Mites Web: a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed Oct 2016
Migeon A, Ferragut F, Escudero-Colomar L, Fiaboe K, Knapp M, de Moraes G, Ueckermann E, Navajas M (2009) Modelling the potential distribution of the invasive tomato red spider mite, Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 48:199–212
Migeon A, Auger P, Hufbauer R, Navajas M (2015) Genetic traits leading to invasion: plasticity in cold hardiness explains current distribution of an invasive agricultural pest, Tetranychus evansi (Acari: Tetranychidae). Biol Invasions 17:2275–2285
Navajas N, de Moraes GJ, Auger P, Migeon A (2013) Review of the invasion of Tetranychus evansi: biology, colonization pathways, potential expansion and prospects for biological control. Exp Appl Acarol 59:43–65
Ortego F (2012) Physiological adaptations of the insect gut to herbivory. In: Smagghe G, Diaz I (eds) Arthropod-plant interactions—novel insights and approaches for IPM. Series: progress in biological control, vol 14. Springer, Berlin, pp 75–88
Rasband WS (1997–2016) ImageJ [Internet]. U. S. National Institutes of Health, Bethesda, MD, USA. https://imagej.nih.gov/ij/
Santamaria ME, González-Cabrera J, Martinez M, Grbic V, Castañera P, Diaz I, Ortego F (2015) Digestive proteases in bodies and faeces of the two-spotted spider mite, Tetranychus urticae. J Insect Physiol 78:69–77
Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Almeida-Oliveira MG, Lima ER, Kant M, Sabelis MW, Janssen A (2011) A herbivore that manipulates plant defence. Ecol Lett 14:229–236
Saunyama IGM, Knapp M (2003) Effects of pruning and trellising of tomatoes on red spider mite incidence and crop yield in Zimbabwe. Afr Crop Sci J 11:269–277
Savalli UM, Fox CW (1998) Sexual selection and the fitness consequences of male body size in the seed beetle Stator limbatus. Anim Behav 55:473–483
Terra WR, Ferreira C (2012) Biochemistry and molecular biology of digestion. In: Gilbert LI (ed) Insect molecular biology and biochemistry. Elsevier, New York, pp 365–418
Van Leeuwen T, Dermauw W (2016) The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu Rev Entomol 61:475–498
Wybouw N, Zhurov V, Martel C, Bruinsma KA, Hendrickx F, Grbić V, van Leeuwen T (2015) Adaptation of a polyphagous herbivore to a novel host plant extensively shapes the transcriptome of herbivore and host. Mol Ecol 24:4647–4663
Ximenez-Embún MG, Ortego F, Castañera P (2016) Drought-stressed tomato plants trigger bottom–up effects on the invasive Tetranychus evansi. PLoS ONE 11:e0145275
Ximénez-Embún MG, Castañera P, Ortego F (2017) Drought stress in tomato increases the performance of adapted and non-adapted strains of Tetranychus urticae. J Insect Physiol 96:73–81
Ziska LH, Blumenthal DM, Runion GB, Hunt ER Jr, Diaz-Soltero H (2011) Invasive species and climate change: an agronomic perspective. Clim Change 105:13–42
Acknowledgements
This work was funded by FACCE Era Net Plus-Food Security, Agriculture, Climate Change Grant (GENOMITE, Proposal No 618105 to P.C., M.N., I.D., A.M. and P.A.), CSIC (Grant CSIC-201040E049 to F.O) and the French Agence Nationale de la Recherche (Grant ANR-14-JFAC-0006-01 to M.N., A.M. and P.A), and it was supported by the metaprogramme Adaptation of Agriculture and Forest to Climate Change (AAFCC) of the French National Institute for Agricultural Research (INRA). Juan de la Cierva 2012 post-doctoral grant subprogram to M.E.S. We are grateful to Prof. Jerry Cross (NIAB EMR, UK) for English editing of the manuscript.
Author’s contributions: FO, PC, ID and MN conceived research. MES, PA, AM and MM designed, performed and analyzed experiments. FO, MN, MES and PA wrote the manuscript. All authors read, corrected and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by A.R. Horowitz.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Distribution of the eggs size (surface) laid by females of each strain (Nice and Perpignan) as a function of rearing host plant (S. nigrum or S. lycopersicum) and test host plant (S. nigrum or S. lycopersicum): A) and B) mites tested on the same host plant on which they were reared, S. lycopersicum and S. nigrum, respectively; C) mites shifted from S. lycopersicum to S. nigrum and vice versa (D) (PDF 312 kb)
Rights and permissions
About this article
Cite this article
Santamaría, M.E., Auger, P., Martínez, M. et al. Host plant use by two distinct lineages of the tomato red spider mite, Tetranychus evansi, differing in their distribution range. J Pest Sci 91, 169–179 (2018). https://doi.org/10.1007/s10340-017-0852-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0852-1