Abstract
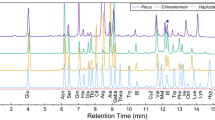
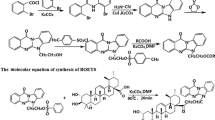
A new fluorescent labeling reagent, benzimidazo[2,1-b]quinazolin-12(6H)-one-5-ethyl-p-toluenesulfonate (BQETS) was designed and synthesized, and it was successfully applied to the determination of fatty acids with liquid chromatography. BQETS can easily and quickly label fatty acids within 20 min at 90 °C in dimethylformamide with K2CO3 as catalyst. The derivatives exhibit high stability and strong fluorescence with excitation and emission wavelengths of 247 and 401 nm, respectively. The 24 derivatives of fatty acids were completely separated by gradient elution on a Hypersil GOLD C18 column. Excellent linear responses for all fatty acids were observed with correlation coefficients of >0.9991. The method also showed good sensitivity and precision, with limits of detection in the 0.0024–0.0206 μg g−1 range and relative standard deviations ≤9.6 %. This is the first time that BQETS fluorescent probe and its applications for the determination of fatty acids have been reported. Moreover, this is the first report on the comparison of free fatty acids composition in the above-ground part of Coriandrum sativum L. from different habitats in China.






Similar content being viewed by others
References
Gou H, Lu Z, Li J (2005) Actuality of the study and development of coriander. Food Res Dev 4:26
Potter TL (1996) Essential oil composition of cilantro. J Agric Food Chem 44:1824–1826
Norman J (1991) The complete book of spices. Viking Press, New York
Kamat A, Pingulkar K, Bhushan B, Gholap A, Thomas P (2003) Potential application of low dose gamma irradiation to improve the microbiological safety of fresh coriander leaves. Food Control 14:529–537
Li W, Chen M (1996) Xi’an Highway Univ 16:88–91
Zhao S (1989) Notebook in valid component of plant drug. Beijing, China
Borges OP, Carvalho JS, Correia PR, Silva AP (2007) Lipid and fatty acid profiles of Castanea sativa Mill. Chestnuts of 17 native Portuguese cultivars. Food Compos Anal 20:80–89
Chen SH, Chuang YJ (2002) Analysis of fatty acids by column liquid chromatography. Anal Chim Acta 465:145–155
Bougnoux P, Hajjaji N, Maheo K, Couet C, Chevalier S (2010) Fatty acids and breast cancer: sensitization to treatments and prevention of metastatic re-growth. Prog Lipid Res 49:76–86
Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A (2004) Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 79:935–945
Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE Jr, Marangell LB, Richardson AJ, Lake J (2006) Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatr 67:1954–1967
Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP (2003) Supplementation with a combination of ω-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res 62:195–204
Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83:S1505–S1519
Mozaffarian D, Wu JH (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58:2047–2067
Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, Carter DC, Fearon KC (1996) The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 12:S27–S30
Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD (2010) Role of fatty acid uptake and fatty acid β-oxidation in mediating insulin resistance in heart and skeletal muscle. BBA-Mol Cell Bioll 1801:1–22
Yang ZH, Miyahara H, Mori T, Doisaki N, Hatanaka A (2011) Beneficial effects of dietary fish-oil-derived monounsaturated fatty acids on metabolic syndrome risk factors and insulin resistance in mice. J Agric Food Chem 59:7482–7489
Bent S, Ko R (2004) Commonly used herbal medicines in the US: a review. Am J Med 116:478–485
Wheat J, Currie G (2008) Herbal medicine for cancer patients: an evidence based review. Int J Altern Med 5:28–30
Takahashi K, Goto-Yamamoto N (2011) Simple method for the simultaneous quantification of medium-chain fatty acids and ethyl hexanoate in alcoholic beverages by gas chromatography-flame ionization detector: development of a direct injection method. J Chromatogr A 1218:7850–7856
Schreiner M (2005) Quantification of long chain polyunsaturated fatty acids by gas chromatography: evaluation of factors affecting accuracy. J Chromatogr A 1095:126–130
Quehenberger O, Armando AM, Dennis EA (2011) High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography–mass spectrometry. BBA Mol Cell Biol 1811:648–656
Manzano P, Diego JC, Nozal MJ, Bernal JL, Bernal J (2012) Gas chromatography–mass spectrometry approach to study fatty acid profiles in fried potato crisps. Food Compos Anal 28:31–39
Brondz I (2002) Development of fatty acid analysis by high-performance liquid chromatography, gas chromatography, and related techniques. Anal Chim Acta 465:1–37
Ruiz-Rodriguez A, Reglero G, Ibañez E (2010) Recent trends in the advanced analysis of bioactive fatty acids. J Pharmaceut Biomed 51:305–326
Sasamot K, Ushijima T, Saito M, Ohkura Y (1996) Precolumn fluorescence derivatization of carboxylic acids using 4-aminomethyl-6, 7-dimethoxycoumarin in a two-phase medium. Anal Sci 12:189–193
You J, Zhang W, Zhang Y (2001) Simple derivatization method for sensitive determination of fatty acids with fluorescence detection by high-performance liquid chromatography using 9-(2-hydroxyethyl)-carbazole as derivatization reagent. Anal Chim Acta 436:163–172
Lu CY, Wu HL, Chen SH, Kou HS (2000) A fluorimetric liquid chromatography for highly sensitive analysis of very long chain fatty acids as naphthoxyethyl derivatives. Chromatographia 51:315–321
Toyo’oka T (2002) Fluorescent tagging of physiologically important carboxylic acids, including fatty acids, for their detection in liquid chromatography. Anal Chim Acta 465:111–130
Li G, You J, Suo Y, Song C, Sun Z, Xia L, Zhao X, Shi J (2011) A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem 125(4):1365–1372
Yang D, Wang Y, Yang H, Liu T, Fu H (2012) Copper-catalyzed domino synthesis of benzimidazo [2, 1-b] quin-azolin-12 (6H)-ones using cyanamide as a building block. Adv Synth Catal 354:477–482
Sun J, Chen G, Zhao X, Xu W, Zhou G, Han Y, You J (2007) Determination of 30 free fatty acids in two famous Tibetan medicines by HPLC with fluorescence detection and mass spectrometric identification. Chromatographia 65:469–476
You J, Zhao X, Suo Y, Li Y, Wang H, Chen G (2007) Determination of long-chain fatty acids in bryophyte plants extracts by HPLC with fluorescence detection and identification with MS. J Chromatogr B 848:283–291
Ruiz-Jiménez J, Priego-Capote F, de Castro ML (2004) Identification and quantification of trans fatty acids in bakery products by gas chromatography–mass spectrometry after dynamic ultrasound-assisted extraction. J Chromatogr A 1045:203–210
Zhang S, You J, Zhou G, Li C, Suo Y (2012) Analysis of free fatty acids in Notopterygium forbesii Boiss by a novel HPLC method with fluorescence detection. Talanta 98:95–100
Ackman R (1998) Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J Am Oil Chem Soc 75:541–545
Yang F, Feng K, Zhao J, Li S (2009) Analysis of sterols and fatty acids in natural and cultured Cordyceps by one-step derivatization followed with gas chromatography–mass spectrometry. J Pharmaceut Biomed 49:1172–1178
Louppis AP, Badeka AV, Katikou P, Paleologos EK, Kontominas MG (2010) Determination of okadaic acid, dinophysistoxin-1 and related esters in Greek mussels using HPLC with fluorometric detection, LC-MS/MS and mouse bioassay. Toxicon 55:724–733
Sun Z, You J, Song C, Xia L (2011) Identification and determination of carboxylic acids in food samples using 2-(2-(anthracen-10-yl)-1H-phenanthro [9, 10-d] imidazol-1-yl) ethyl 4-methylbenzenesulfonate (APIETS) as labeling reagent by HPLC with FLD and APCI/MS. Talanta 85:1088–1099
Acknowledgments
The research was supported by the National Natural Science Foundation of China (Nos. 21275089, 21475075).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sun, Y., Zhang, X., Ji, Z. et al. Determination of Free Fatty Acids of Chinese Coriandrum sativum L. Using Benzimidazo[2,1-b]quinazolin-12(6H)-one-5-ethyl-p-toluenesulfonate as Precolumn Labeling Reagent by LC with Fluorescence Detection. Chromatographia 79, 547–559 (2016). https://doi.org/10.1007/s10337-016-3071-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3071-7