Abstract
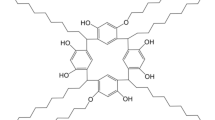
In this paper, the effects of functionalization with terpenes on two new liquid-crystalline stationary phases for gas chromatography (GC) are described. Citronellol was used as the terminal group in the first material, and tetrahydrogeraniol was used with a second material. Inverse GC showed that the new materials have wide liquid-crystalline ranges (mesophases), 371–500 and 395–501 K, respectively. Moreover, they show good thermal stability up to 523 K and good potential as stationary phases for capillary GC. To clarify the effects of the liquid crystal structures and functional groups on retention and separation, the chromatographic behaviors of the two stationary phases were compared by eluting alkylbenzenes, polycyclic aromatic hydrocarbons, aromatic compounds, and terpenoids. The selectivities for a wide range of analytes achieved using the citronellol column were significantly better than those obtained using the tetrahydrogeraniol column. The columns showed different retention behaviors and fine resolutions for some of the main constituents of essential oils. Introduction of the double bond of citronellol greatly improved the polarization interactions involved in the shape recognition of the liquid-crystalline state for isomers. The new citronellol liquid-crystalline stationary phase, therefore, has a high affinity for natural compounds.







Similar content being viewed by others
References
Kelker H (1963) Kristallin-flüssige Schmelzen als stationäre Phasen in der Gas-Flüssigkeits-Verteilungschromatographie. Z Anal Chem 198(3):254–266. doi:10.1007/BF00486441
Emad Ghanem SA-H (2014) Separation of isomers on nematic liquid crystal stationary phases in gas chromatography: a review. Chromatographia 77(9–10):653–662. doi:10.1007/s10337-014-2675-z
Ferroukhi O, Atik N, Guermouche S, Guermouche MH, Berdagué P, Judenstein P, Bayle JP (2000) High performance liquid chromatography of aromatic and polyaromatic hydrocarbons on a new chemically bonded liquid crystal phase. Chromatographia 52(9–10):564–568. doi:10.1007/BF02789751
Witkiewicz Z (1989) Application of liquid crystals in chromatography. J Chromatogr A 466:37–87. doi:10.1016/S0021-9673(01)84616-1
Witkiewicz Z, Oszczudłowski J, Repelewicz M (2005) Liquid-crystalline stationary phases for gas chromatography. J Chromatogr A 1062(2):155–174. doi:10.1016/j.chroma.2004.11.042
Demus D, Goodby JW, Gray GW, Spiess HW, Vill V (1998) Handbook of liquid crystals fundamentals. Wiley, VCH Verlag GmbH, USA, pp 133–187
Berdagué P, Perez F, Courtieu J, Bayle JP, Abdelhadi O, Guermouche S, Guermouche MH (1995) Gas chromatographic study of the thermal and analytical properties of a nematic liquid crystal and its cupric complex. J High Resolut Chromatogr 18(5):304–308. doi:10.1002/jhrc.1240180508
Witkiewicz Z, Wacławczyk A (1979) Some properties of high-temperature liquid crystalline stationary phases. J Chromatogr A 173(1):43–52. doi:10.1016/S0021-9673(01)80444-1
Dahmane M, Athman F, Sebih S, Guermouche M-H, Bayle J-P, Boudah S (2010) Effect of the chain length on the thermal and analytical properties of laterally biforked nematogens. J Chromatogr A 1217(42):6562–6568. doi:10.1016/j.chroma.2010.08.036
Addoun A, Ferroukhi O, Dahmane M, Guermouche S, Bayle JP, Guermouche M (2014) Three nematogen azobenzene-based stationary phases for capillary GC: synthesis and comparative study. Chromatographia 77(19–20):1367–1377. doi:10.1007/s10337-014-2743-4
Ammar-Khodja F, Guermouche S, Guermouche MH, Berdagué P, Bayle JP (1999) Gas chromatographic properties of some liquid crystals containing dioxyethylene ether terminal chains. Chromatographia 50(5–6):338–345. doi:10.1007/BF02490839
Chester TL, Coym JW (2003) Effect of phase ratio on van’t Hoff analysis in reversed-phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy. J Chromatogr A 1003:101–111. doi:10.1016/S0021-9673(03)00846-X
Price GJ, Hickling SJ, Shillcock IM (2002) Applications of inverse gas chromatography in the study of liquid crystalline stationary phases. J Chromatogr A 969(1–2):193–205. doi:10.1016/S0021-9673(02)00889-0
Llorente MA, Menduiña C, Horta A (1979) Inverse gas chromatography of poly(cyclohexyl methacrylate). J Polym Sci 17(2):189–197. doi:10.1002/pol.1979.180170201
Gritti F, Félix G, Achard MF, Hardouin F (2000) Investigation of the nematic—isotropic transition of a liquid crystalline polymer and determination of molecular diffusion coefficients using gas chromatography. J Chromatogr A 893(2):359–366. doi:10.1016/S0021-9673(00)00772-X
Reid VR, Crank JA, Armstrong DW, Synovec RE (2008) Characterization and utilization of a novel triflate ionic liquid stationary phase for use in comprehensive two-dimensional gas chromatography. J Sep Sci 31(19):3429–3436. doi:10.1002/jssc.200800251
Kelker H, VS E (1968) Advances in chromatography, vol 6. Marcel Dekker, New York
Schroeder J (1974) Scientific and technological application of liquid crystals. In: G.W. G, P.A. W (eds) Liquid crystals and plastic crystals: preparation, constitution and applications, vol 1. E. Horwood, New York
Sun X, Zhu Y, Wang P, Li J, Wu C, Xing J (2011) High temperature and highly selective stationary phases of ionic liquid bonded polysiloxanes for gas chromatography. J Chromatogr A 1218(6):833–841. doi:10.1016/j.chroma.2010.12.036
Breckler PN, Betts TJ (1970) Relative retention time changes with temperature for the gas chromatographic identification of volatile oil components. J Chromatogr A 53(2):163–170. doi:10.1016/S0021-9673(01)98456-0
Boudah S, Sebih S, Guermouche MH, Rogalski M, Bayle JP (2003) Thermal properties and chromatographic behavior of a mixture of two liquid crystals. Chromatographia 57(1):S307–S311. doi:10.1007/BF02492121
Athman F, Dahmane M, Boudah S, Guermouche M-H, Bayle J, Sebih S (2009) Evaluation of thermal and analytical properties of two liquid crystals in capillary GC. Chromatographia 70(3–4):503–510. doi:10.1365/s10337-009-1212-y
Belaïdi D, Sebih S, Guermouche MH, Bayle JP, Boudah S (2003) Comparison of two liquid crystals as stationary phases in capillary gas chromatography. Chromatographia 57(3–4):207–212. doi:10.1007/BF02491718
Ghatge BB, Bhalerao NV (1991) Application of substituted liquid crystals as stationary phases in gas-liquid chromatography for the separation of mono-and dimethyl naphthalenes. J Chromatogr A 549:423–428. doi:10.1016/S0021-9673(00)91454-7
Betts TJ, Finucane GJ, Tweedie HA (1981) Practical system for polarity rating of packed gas-liquid chromatography columns. J Chromatogr A 213(2):317–322. doi:10.1016/S0021-9673(00)81914-7
Qiao L, Lu K, Qi M, Fu R (2013) Separation performance of guanidinium-based ionic liquids as stationary phases for gas chromatography. J Chromatogr A 1276:112–119. doi:10.1016/j.chroma.2012.12.039
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferroukhi, O., Addoun, A., Guermouche, S. et al. Evaluation of the Effect of Citronellol Group on Functionalized Mesogenic Materials by Capillary GC. Chromatographia 78, 1251–1261 (2015). https://doi.org/10.1007/s10337-015-2938-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-015-2938-3