Abstract
Breeding performance of many vertebrate species is known to improve over the early stages of the life cycle, which has been commonly attributed to the progressive improvement in competences and increasing investment in reproduction. While there is a large body of evidence for age-related variation in fecundity within bird populations, much less is known on whether and how age affects other traits related to reproduction. The aim of this study was to test for age-related patterns in fecundity, nest-site location, offspring condition and brood sex ratio in a long-lived colonial waterbird, the Great Cormorant (Phalacrocorax carbo sinensis). For this purpose we collected data on 180 breeding attempts of 100 Great Cormorants aged two to 11 years. We found a progressive increase in reproductive output with age and we showed that older individuals nested closer to the colony centre and in higher nesting densities. We also found that parental age was positively related to physiological condition of offspring (measured with whole-blood haemoglobin concentration and plasma glucose concentration). There was no indication for age-related adjustments in brood sex ratio in our cormorant population. Our results indicate a great complexity of the processes which produce age-related improvements in reproductive performance within avian populations.
Zusammenfassung
Einfluss des Alters auf Reproduktionserfolg, Neststandort und Kondition der Jungvögel beim Kormoran
Phalacrocorax carbo sinensis Es ist bekannt, dass sich die Brutleistung vieler Wirbeltierarten während der frühen Lebensabschnitte steigert, was üblicherweise der zunehmenden Verbesserung von Fähigkeiten und der wachsenden Investition in die Fortpflanzung zugeschrieben wird. Zwar gibt es eine Menge Hinweise auf altersbedingte Variation der Fruchtbarkeit innerhalb von Vogelpopulationen, deutlich weniger ist aber darüber bekannt, ob und wie das Alter sich auf andere reproduktionsbezogene Merkmale auswirkt. Das Ziel dieser Studie war es, nach altersabhängigen Mustern in der Fruchtbarkeit, der Nistplatzwahl, der Jungvogelkondition sowie des Geschlechterverhältnisses der Bruten bei einem langlebigen, koloniebrütenden Wasservogel, dem Kormoran (Phalacrocorax carbo sinensis) zu suchen. Zu diesem Zweck sammelten wir Daten aus 180 Brutversuchen von 100 Kormoranen im Alter zwischen zwei und elf Jahren. Wir stellten eine zunehmenden Anstieg der Reproduktionsleistung mit dem Alter fest und konnten zeigen, dass ältere Individuen näher am Koloniezentrum und in höherer Dichte nisteten. Außerdem entdeckten wir, dass das Alter der Elternvögel in positivem Zusammenhang mit der Körperkondition (gemessen an der Vollblut-Hämoglobinkonzentration und dem Plasma-Glukosespiegel) der Nachkommen stand. Es gab in unserer Kormoranpopulation keine Anzeichen für altersbedingte Anpassungen des Geschlechterverhältnisses der Bruten. Unsere Ergebnisse deuten auf eine hohe Komplexität der Prozesse hin, welche die altersabhängige Verbesserung der Reproduktionsleistung in Vogelpopulationen bewirken.
Similar content being viewed by others
Introduction
Age-related variation in reproductive performance is a widespread phenomenon in animals, including birds (Forslund and Pärt 1995). It has been documented in many avian taxa that reproductive performance considerably improves with age, especially during the early stages of the life cycle (Reid et al. 2003; Blas et al. 2009; Nisbet and Dann 2009). This initial increase in reproductive performance has been commonly attributed to three non-exclusive processes: (1) experience-related improvement in breeding skills such as nest-site choice, nest construction, or incubation (Cichoń 2003); (2) progressive improvement in skills that are not directly linked to reproduction, but are likely to have a positive impact on reproductive output, e.g., foraging efficiency and social skills (Desrochers 1992); (3) increasing investment in reproduction, as young individuals are more likely to trade current investment in reproduction for future survival (Curio 1983).
While there is a large body of evidence for age-related variation in fecundity in bird populations (Croxall et al. 1990; Robertson and Rendell 2001; Reid et al. 2003), much less is known on how nest-site selection patterns change with age. The choice of optimal microhabitat or nest sites is often crucial for brood survival, and thus settlement decisions largely determine reproductive output of individuals (Møller 1989; Fisher and Wiebe 2006). Since birds rely on their prior experience during nest-site selection (e.g., Marzluff 1988), older individuals should have better capabilities to reliably assess the quality of nest sites and choose those which are less vulnerable to predation, parasitism, or adverse weather conditions. However, in most natural bird populations individuals do not have an unlimited access to high-quality territories that provide a large fitness payoff. Consequently, there is usually an intense competition for the most profitable nest sites and large competitive asymmetries between individuals might produce an ideal despotic distribution of individuals in space (Fretwell 1972), where dominant individuals secure the best territories or nest sites and relegate subordinate conspecifics to less attractive locations. As social rank is known to increase with age, older individuals are likely to be dominant over younger ones (Holberton et al. 1990), and thus should have priority access to high-quality nest sites. In migratory birds, age differences in nest-site selection may also be mediated by quality-dependent timing of arrival at breeding grounds. Older migrants are likely to arrive on breeding grounds earlier, when many attractive territories are still available, while nest-site choice by younger late-arriving conspecifics is constrained by the choice of previously established males (Lozano et al. 1996).
Age of individuals is also expected to affect the level of investment in offspring (Forslund and Pärt 1995). In size dimorphic species, younger individuals are likely to adjust their brood sex ratio towards offspring of the smaller sex, which is less costly to rear (Blank and Nolan 1983; Heg et al. 2000). As younger individuals usually have reduced foraging efficiency, their food delivery rates for offspring are lower when compared with older conspecifics (Daunt et al. 2007). Younger parents may also be less synchronized in food provisioning, which may entail negative consequences primarily for offspring of the more energy-demanding, larger sex (Weimerskirch et al. 2000). Thus, producing broods biased towards the smaller sex is likely to maximize brood survival and offspring quality in young inexperienced pairs (Daunt et al. 2001). Under limited possibilities for adaptive brood sex ratio adjustments, a negative relationship between parental age and offspring condition or growth rate might be expected (Pugasek 1993).
In this study, we aimed to test for age-related patterns in fecundity, nest site location, brood sex ratio, and offspring condition in a long-lived (max. lifespan around 20 years; Nelson 2005) colonial waterbird, the Great Cormorant Phalacrocorax carbo sinensis. For this purpose, we collected data on reproductive performance of Great Cormorants aged 2–11 years—a period that precedes a senescent decline in this species (Bregnballe 2006). While age-related differences in breeding success have already been described for the Great Cormorant (Bregnballe 2006), there is no information on how other reproductive components (brood sex ratio, offspring condition) vary with age in this species. Also, we are aware of no other studies investigating age effects on nest location in the Great Cormorant, despite the fact that nest-site selection can have profound influence on population structure and dynamics (Thompsoan and Furness 1991). To fill these gaps we tested four specific hypotheses on the age-related patterns in the Great Cormorant:
-
1.
Older individuals should have higher fledging success via progressive improvement in breeding and non-breeding skills, as well as increasing reproductive investment.
-
2.
Older individuals should nest in higher nesting densities, closer to the colony centre, and higher above the ground. Densely-occupied, central parts of colonies are generally more attractive, as they are less accessible to predators (Götmark and Andersson 1984), and thus are expected to be occupied by older, dominant individuals. Similarly, older individuals should be able to secure nest sites located higher above the ground, which may also increase brood safety (Post 1990).
-
3.
Older individuals should produce more male than female offspring. Male cormorants are on average 10 % larger and 20 % heavier than females (Koffijberg & Van Eerden 1995, Liordos & Goutner 2008), which means that female offspring require lower parental food investment. Thus, young pairs which are likely to forage less efficiently should produce female-biased broods.
-
4.
Older individuals should produce offspring in better condition. Older parents are expected to have increased food delivery rates via progressive improvement in individual foraging skills and increasing reproductive investment.
To test hypotheses one and two, we collected data on the reproductive output and within-colony nest-site position for 180 breeding attempts by 100 different known-age cormorants. To test hypotheses three and four we molecularly determined sex and measured three condition parameters (size-adjusted body mass, whole-blood haemoglobin concentration, and plasma glucose concentration) in nearly 130 cormorant chicks from 41 broods raised by at least one known-age parent.
Methods
Study area and general field procedures
The study was conducted in a colony of Great Cormorants at the Jeziorsko reservoir (51°73′N, 18°63′E), central Poland. The colony was established in 1991, when 90 breeding pairs were recorded at the site. Since then, the size of the colony gradually increased till 2013, when ca. 800 pairs bred at the reservoir. Afterwards, in 2014–2015 the colony size dropped to ca. 500 pairs due to unfavourable water-level conditions at the reservoir. Although the colony has changed location several times since establishment, spatial organization of the colony remained relatively unchanged during the main study period (2012–2015). The structure of the colony was relatively regular, with a well-defined central part characterized by highest nesting densities (Fig. 1). The colony was located in an area covered with riparian willow woodland dominated by the white willow Salix alba and the grey willow Salix cinerea. All nests were located on willows at the height of 4–16 m above the ground. The colony was located within the borders of Jeziorsko nature reserve, where human disturbance was negligible. Cormorants from the studied population are fully migratory; they arrive at the colony over several weeks in March and April.
Since 2004, 2396 Great Cormorant nestlings were ringed in the colony, 87.1 % of which were additionally marked with an engraved colour plastic leg band. Colour bands ensured higher readability and could be read with a 20–60x scope from a distance of up to 400 m. In 2012–2015, we searched for marked individuals that returned to the colony for breeding. Resighting effort was constant between years and 41–55 marked individuals were resighted and assigned to nests each year. In total, we resighted 100 different individuals, but 44 of them were resighted in more than one breeding season, resulting in a total number of 180 resightings associated with different breeding attempts. For most of these breeding attempts (93.9 %, n = 180) we collected information on the number of young fledged per initiated clutch (number of young at the age of ca. 1 month), henceforth referred to as reproductive success.
Nest-site location and nesting density
We used a hand held Global Positioning System (GPS) unit (Garmin GpsMap 60Cx, Olathe, KS, USA) with European Geostationary Navigation Overlay Service (EGNOS) ensuring accuracy of 1–1.5 m to map all active nests each year. Collected coordinates were used to calculate distances between all the nests in the colony. Using the nest-distance matrices we calculated nesting density (number of active nests within a radius of 20 m) and distance to the colony centre (m) for each nest. The centre of the colony was calculated as the mean coordinates of all active nests within the colony in a given year. There was a significant negative correlation between nesting density and distance to the colony centre, indicating that central parts of the colony were associated with higher densities (−0.41 < r < −0.16 in different years, all P < 0.001). As the strength of these correlations was moderate, both nesting density and distance to the colony centre could be fitted as independent variables into one model without violating the assumption of little/no multicollinearity (|r| < 0.8) in the data. We also collected data on nest height, measured as a distance from the ground to the bottom of the nest (±0.5 m).
Blood sampling and offspring measurements
For 41 broods of different resighted individuals we collected offspring blood to measure physiological condition and determine sex with molecular techniques (n = 129 offspring). Ca. 1 ml of blood was collected from the brachial vein of each nestling. 20 μl of blood was stored in 96 % ethanol for molecular sexing and 5 μl was used to measure whole-blood haemoglobin concentration. Remaining blood was collected into ice-cooled EDTA tubes and centrifuged at 3000 rpm for 15 min within eight hours of collection. Plasma was separated from blood cells and kept at −20° C until analysis of glucose concentrations. All blood samples were collected between 8:00 and 13:00. Upon blood sampling, we also collected the following measurements: wing length (±1 mm), head length, bill length, and tarsus length (both ± 0.1 mm). Chicks were also weighed (±1 g) using an electronic scale.
Offspring condition
We used body mass adjusted for structural size as the first body condition index. To derive this estimate we used the scaled mass index recommended by Peig and Green (2009), which was calculated according to the following equation:
where M i and L i are the body mass and the linear body measurement of individual i respectively; b SMA is the scaling exponent estimated by the standardized major axis regression of body mass on linear body measurement; L 0 is the mean value of the linear body measurement for the study population; and \(\widehat{M}_{i}\) is the predicted body mass for individual i when the linear body measure is standardized to L 0 . We used bill length as a linear body measurement, as it showed the strongest correlation with body mass on a log–log scale among all collected biometrical traits (r = 0.93, n = 129, P < 0.001), indicating that it best explained fraction of mass associated with structural size (Peig and Green 2009). b SMA was estimated with RMA software (Bohonak 2004) using 100,000 bootstraps.
Physiological condition of chicks was assessed with the whole-blood haemoglobin concentration and plasma glucose concentration. The concentration of haemoglobin is the most important determinant of blood oxygen-carrying capacity; a low level of this protein is associated with a detrimental state of anemia. In birds, haemoglobin concentration has been reported to correlate with size-corrected body mass (Lill et al. 2013), diet quality (Pryke and Rollins 2012), parasitic rates (Słomczyński et al. 2006), hatching date (Minias et al. 2015), and survival (Bańbura et al. 2007), giving strong support for reliability of this parameter as a measure of individual quality (reviewed in Minias 2015). The concentration of haemoglobin was determined using a portable HemoCue Hb 201+photometer (HemoCue Hb, Ängelholm, Sweden), which is acknowledged to reliably measure hemoglobin concentration in avian blood (Velguth et al. 2010).
Plasma glucose concentrations have also been widely used to assess nutritional state in different animal species, including birds (e.g., Jenni-Eiermann and Jenni 1994). Reduced plasma glucose turnover was recorded during fasting in birds (Groscolas and Rodriguez 1981) and low concentrations of plasma glucose under natural starvation have been reported for several avian taxa (González and Hiraldo 1991; Jenni-Eiermann and Jenni 1997; Ferrer and Dobado-Berrios 1998). Finally, among several different plasma metabolites the concentration of plasma glucose was found to be the most reliable indicator of body condition in Great Cormorant nestlings (Minias and Kaczmarek 2013a). Plasma glucose concentration was measured using commercial kits and reagents (glucose oxidase/peroxidase reaction) with a spectrophotometer (BTS-330, BioSystems Reagents & Instruments, Barcelona, Spain). Plasma glucose concentration positively correlated with blood haemoglobin concentration (r = 0.33, n = 129, P < 0.001), while neither of these two parameters correlated with the scaled mass index (all P > 0.05).
Molecular sexing
All nestlings (n = 129) were molecularly sexed. Nuclear DNA from blood samples was extracted with the Thermo Scientific Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the kit protocol. Amplification of the chromo-helicase-DNA-binding (CHD) region was performed with the primer pair 2550F and 2718R (Fridolfsson and Ellegren 1999), according to the protocol described by Griffiths et al. (1998). The PCR products were stained and separated on 2 % agarose gel until the differences in the product size (ca. 200 bp) were clearly visible. Offspring sex ratio was expressed as the percentage of males in a brood.
Statistical analyses
We used general linear mixed models to analyse age effects on nest-site location, reproductive success, and offspring condition of Great Cormorants. In the analyses of nest-site location and reproductive success, the identity of birds was entered as a random effect in each model to avoid pseudoreplication resulting from repeated measures of the same individuals. In the analyses of offspring condition, the identity of brood was entered as a random effect in each model, because data from siblings were non-independent. Distance to the colony centre and nestling blood haemoglobin concentration were log-transformed, while nestling plasma glucose concentration was squared to improve normality before analysis. To analyse age effects on offspring sex ratio we used a generalized linear mixed model with binomial distribution and logit link function with brood identity entered as a random factor. In all models we also entered the squared term of age to test for non-linear age effects. To select for significant predictor variables, the stepwise procedures of backward removal were used. The strength and character of significant relationships were evaluated with β coefficients. Effect sizes were measured with partial eta-squared (η2). All values are presented as mean ± SE. All analyses were performed with JMP Pro 10 (SAS Institute Inc., Cary, NC, USA).
Results
The age of breeding cormorants varied between 2 and 11 years. We recorded many fewer 2-year old than 3-year old individuals (9.0 vs. 35.0 %), suggesting that most individuals do not attempt breeding at the age of two (Fig. 2). We recorded only two individuals breeding at the age of eleven.
We found that older birds bred in higher nesting densities (F 1,79 = 4.18, P = 0.043, β = 0.080 ± 0.039) and closer to the centre of the colony (F 1,79 = 3.92, P = 0.049, β = −0.075 ± 0.037; Fig. 3a). Squared terms of adult age were non-significant and excluded from both models (nesting density: F 1,78 = 0.04, P = 0.84; distance to the colony centre: F 1,78 = 0.02, P = 0.90). There was no relationship between adult age and nest height (F 1,79 = 1.75, P = 0.19; Fig. 3b).
The mean reproductive success was 2.51 ± 0.10 and 85.8 % of breeding attempts were successful (at least one fledgling raised). We found a significant linear relationship between the age of adult birds and their reproductive success (F 1,72 = 4.18, P = 0.044), as older individuals fledged more young (β = 0.102 ± 0.050; Fig. 4). The squared term of adult age was non-significant and excluded from the model (F 1,71 = 1.61, P = 0.21). When we controlled for the variation in the nest location (nesting density and distance to the colony centre), the positive effect of adult age on reproductive success was nearly significant (F 1,70 = 3.86, P = 0.053; β = 0.099 ± 0.050). Age explained 5.5 % of variance in reproductive success, while nest location explained no additional variance (<1 % for nesting density and distance to colony centre).
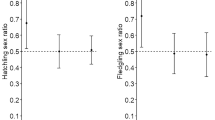
Condition of offspring also depended on the age of parents. After controlling for the effects of date and hour of sampling, we found significant linear relationships of parental age with whole-blood haemoglobin concentration (F 1,88 = 5.63, P = 0.024, β = 0.157 ± 0.066; Fig. 5a) and plasma glucose concentration (F 1,87 = 4.21, P = 0.048, β = 0.124 ± 0.060; Fig. 5b) of nestlings. The effect of nestling sex was controlled for only in the analysis of glucose concentrations, as males had significantly higher glucose concentrations than females (361.0 vs. 336.6 mg/dl; F 1,87 = 5.92, P = 0.017). By contrast, there was no between-sex variation in haemoglobin concentration (F 1,87 = 0.21, P = 0.64), so the effect of sex was excluded from the model. Squared terms of parental age were non-significant and excluded from both models (haemoglobin concentration: F 1,87 = 0.22, P = 0.64; glucose concentration: F 1,87 = 0.02, P = 0.90). We found no effect of parental age on the scaled mass index of nestlings (F 1,87 = 0.11, P = 0.74; Fig. 5c). Similarly, there was no significant effect of parental age on offspring sex ratio (W = 0.042, P = 0.97; Fig. 6).
Discussion
In this study, we provided evidence for age-related patterns in fecundity and nest-site selection in the Great Cormorant. Specifically, we found a progressive increase in reproductive output with age and we showed that older individuals were able to secure the most attractive, central nest sites in the colony. We also found that parental age was positively related to physiological condition of offspring, although there was no indication for age-related adjustments in brood sex ratio in our cormorant population.
To date, there is limited evidence on how age affects nest-site selection patterns in natural bird populations. It is generally assumed that older individuals are likely to get better access to different resources (including nest sites) through improvements in social skills and dominance status (Piper 1997). Older individuals may develop plumage characters that signal dominance, which produces a well–established age hierarchy (Fugle et al. 1984). In addition, older individuals should progressively gain experience in dominance interactions during their lifetimes, which may improve their ability to dominate opponents (Arcese and Smith 1985). Great Cormorants are known to actively compete for nesting sites using aggressive vocalization and dominance postures (Kortland 1995). Assuming that older cormorants become more dominant over younger conspecifics, they should be able to secure high-quality nest sites within the colony. Also, older birds are likely to arrive earlier at breeding grounds, and thus have a priority access to nest sites of superior quality. It has been shown that old Great Cormorant males (8–12 years old) arrived at the Vørso colony, Denmark, on average 14 days earlier than 2–3 year old males, and thus were more likely to choose the most attractive nest sites (Bregnballe 2006).
In colonies which have a well-developed and inter-annually stable central-periphery structure, selecting an expected central nest site early in the season may convey large fitness benefits (Minias 2014). It has been commonly reported that pairs nesting in the centres are likely to achieve higher breeding success due to limited predatory pressure (Götmark and Andersson 1984; Antolos et al. 2006). The mechanisms explaining lower susceptibility of central pairs to predation may include reduced accessibility for predators (Yorio and Quintana 1997), but also more efficient detection and deterrence of predators in colony centres (Elliot 1985; Roberts 1996). Higher effectiveness of group defence in central parts of the colonies may also be associated with higher breeding densities. Consistently with these predictions, predation-related brood losses have been reported to positively correlate with nearest neighbour distance in the studied Great Cormorant colony at Jeziorsko reservoir (Minias and Kaczmarek 2013b), as either: (1) dense clusters of breeding cormorants were likely to more effectively defend their broods against predators; (2) avian predators (mostly corvids) preferably foraged in the peripheral parts of the colony, where they searched for isolated unattended nests (PM, pers. observation). As predation was one of the major causes of breeding failures in the colony (Minias and Kaczmarek 2013b), the choice of central sites by older, early-arriving individual could contribute to their high reproductive success, although we found that nest location explained no additional variance in reproductive success, when we accounted for age differences. We did not find any relationship between nest height and age, suggesting that locating a nest high in the tree may be of limited benefit for adult individuals, at least in the studied colony. So far, the evidence for age-related segregation of birds within avian colonies is scarce and have been mostly collected for Larids. Exclusion of young individuals from colony centres has been reported for example in ring-billed gulls Larus delawarensis (Haymes and Blokpoel 1980) and California gulls L. californicus (Pugasek and Diem 1983). In the Great Cormorant, age-related spatial segregation has been found at a post-breeding roost, where older individuals occupied places higher in the trees where they were safer from human intruders (Galván 2004).
After controlling for the effect of nest-site location, we found a nearly significant age-related increase in the fledging success of cormorants. Consistently, we found that physiological condition of offspring measured with blood haemoglobin concentration and plasma glucose concentration was positively related to parental age. Both these relationships likely indicate a progressive improvement in breeding-related skills and/or higher investment in offspring by older individuals. While increasing fledging success may be a result of experience-related improvement in certain reproductive behaviours, such as nest construction, incubation, or nest protection (Bregnballe 2006), better condition of chicks is probably associated with increasing foraging efficiency of parents. In fact, there is empirical evidence for increasing foraging performance with age in different cormorant species (Morrison et al. 1978; Daunt et al. 2007), and a similar mechanism has been previously proposed to account for age-related increase in fecundity of the Great Cormorant (Bregnballe and Gregersen 1997).
On the other hand, not only individual processes may contribute to age-related increase in reproductive performance that is observed at the population level. First, it is expected that poor-quality individuals die at younger ages, which produces a progressive disappearance of poor-quality phenotypes (Blas et al. 2009). Second, high-quality individuals may delay breeding to avoid costs of reproduction at younger ages, which may produce a progressive recruitment of good breeders into the population (Lessells and Krebs 1989). In fact, our data suggest that only a minor fraction of 2 year old individuals attempted breeding, and the process of recruitment in the Great Cormorant has been shown to last until the age of 8 years (Frederiksen and Bregnballe 2001). Thus, both these mechanisms are expected to increase mean reproductive output in successive age classes within populations. However, previous studies of the Great Cormorant colony at Vørso, Denmark, revealed that within-individual improvements in competences explained 70–90 % of the age-effect in fecundity observed within the population over the youngest ages, while the progressive disappearance of phenotypes was of secondary importance (Bregnballe 2006). It remains to be established whether population processes could also account for the differences in nest-site selection and offspring condition that we observed between age classes.
Our study provided no evidence for age-related adjustments in brood sex ratio in the Great Cormorant. This result may seem surprising, considering that adaptive manipulations of brood sex ratio at the primary level have already been described in the same population of Great Cormorants. It has been shown by Wojczulanis-Jakubas et al. (2013) that cormorant pairs which nested late in the breeding season and were of supposedly low quality produced broods biased towards offspring of the smaller sex (females). These adjustments were found adaptive, as male chicks had significantly lower body mass if they hatched later in the season, whereas there was no such relationship for female offspring (Wojczulanis-Jakubas et al. 2013). However, we cannot exclude that these kind of adaptations are likely to occur only under poor feeding conditions, and lack of evidence for age-effects in brood sex ratio may be attributed to relatively high food availability during the study period. This explanation seems likely, considering that the level of sexual size dimorphism in the Great Cormorant is relatively low (10–20 % difference in size and mass; Liordos & Goutner 2008) and resulting differences in energy requirements between male and female offspring may easily become unimportant when food is plentiful.
In conclusion, our results indicate a great complexity of the processes which produce age-related improvements in fecundity within avian populations. It has to be kept in mind, though, that our results were based on relatively small sample sizes and the study period was relatively short for investigating age effects in a long-lived species, so we encourage more empirical studies on age-related patterns in reproductive traits of tree-nesting Great Cormorants.
References
Antolos M, Roby DD, Lyons DE, Anderson SK, Collins K (2006) Effects of nest density, location and timing of breeding success of Caspian Terns. Waterbirds 29:465–472
Arcese P, Smith JNM (1985) Phenotypic correlates and ecological consequences of dominance in song sparrows. J Anim Ecol 54:817–830
Bańbura J, Bańbura M, Kaliński A, Skwarska J, Słomczyński R, Wawrzyniak J, Zieliński P (2007) Habitat and year-to-year variation in haemoglobin concentration in nestling blue tits Cyanistes caeruleus. Comp Biochem Physiol A 148:572–577
Blank JL, Nolan V Jr (1983) Offspring sex ratio in red-winged blackbirds is dependent on maternal age. Proc Natl Acad Sci USA 80:6141–6145
Blas J, Sergio F, Hiraldo F (2009) Age-related improvement in reproductive performance in a long-lived raptor: a cross‐sectional and longitudinal study. Ecography 32:647–657
Bohonak AJ (2004) RMA: software for reduced major axis regression. http://www.bio.sdsu.edu/pub/andy/RMA.html. Accessed 26 July 2016
Bregnballe T (2006) Age-related fledgling production in great cormorants Phalacrocorax carbo: influences of individual competence and disappearance of phenotypes. J Avian Biol 37:149–157
Bregnballe T, Gregersen J (1997) Age-related reproductive success in cormorant Phalacrocorax carbo. Pol J Ecol 45:127–135
Cichoń M (2003) Does prior breeding experience improve reproductive success in collared flycatcher females? Oecologia 134:78–81
Croxall JP, Rothery P, Pickering SPC, Prince PA (1990) Reproductive performance, recruitment and survival of wandering albatrosses Diomedea exulans at Bird Island, South Georgia. J Anim Ecol 59:775–796
Curio E (1983) Why do young birds reproduce less well? Ibis 125:400–404
Daunt F, Monaghan P, Wanless S, Harris MP, Griffiths R (2001) Sons and daughters: age-specific differences in parental rearing capacities. Funct Ecol 15:211–216
Daunt F, Wanless S, Harris MP, Money L, Monaghan P (2007) Older and wiser: improvements in breeding success are linked to better foraging performance in European shags. Funct Ecol 21:561–567
Desrochers A (1992) Age and foraging success in the European blackbirds: variation between and within individuals. Anim Behav 43:885–894
Elliot RD (1985) The exclusion of avian predators from aggregations of nesting lapwings (Vanellus vanellus). Anim Behav 33:308–314
Ferrer M, Dobado-Berrios P (1998) Factors affecting plasma chemistry values of the Spanish Imperial Eagle, Aquila adalberti. Comp Biochem Physiol A 120:209–217
Fisher RJ, Wiebe KL (2006) Nest site attributes and temporal patterns of northern flicker nest loss: effects of predation and competition. Oecologia 147:744–753
Forslund P, Pärt T (1995) Age and reproduction in birds—hypotheses and tests. Trend Ecol Evol 10:374–378
Frederiksen M, Bregnballe T (2001) Conspecific reproductive success affects age of recruitment in a great cormorant, Phalacrocorax carbo sinensis, colony. Proc R Soc Lond B 268:1519–1526
Fretwell SD (1972) Populations in seasonal environments. Princeton University Press, Princeton
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non ratite birds. J Avian Biol 30:116–121
Fugle GN, Rothstein SI, Osenberg CW, McGinley MA (1984) Signals of status in wintering white-crowned sparrows. Zonotrichia leucophrys gambelii. Anim Behav 32(IN1):87–93
Galván I (2004) Age-related spatial segregation of great cormorants in a roost. Waterbirds 27:377–381
González JL, Hiraldo F (1991) Some hematological data from marsh harriers (Circus aeruginosus) in central Spain. Comp Biochem Physiol A 100:735–737
Götmark F, Andersson M (1984) Colonial breeding reduces nest predation in the Common Gull (Larus canus). Anim Behav 32:485–492
Griffiths R, Double MC, Orr K, Dawson RJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Groscolas R, Rodriguez A (1981) Glucose metabolism in fed and fasting emperor penguins (Aptenodytes forsteri). Comp Biochem Physiol A 70:191–198
Haymes GT, Blokpoel H (1980) The influence of age on breeding biology of Ring-billed Gulls. Wilson Bull 92:221–228
Heg D, Dingemanse NJ, Lessells CM, Mateman AC (2000) Parental correlates of offspring sex ratio in Eurasian Oystercatchers. Auk 117:980–986
Holberton RL, Hanano R, Able KP (1990) Age-related dominance in male dark-eyed juncos: effects of plumage and prior residence. Anim Behav 40:573–579
Jenni-Eiermann S, Jenni L (1994) Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the Garden Warbler. Auk 111:886–897
Jenni-Eiermann S, Jenni L (1997) Diurnal variation of metabolic responses to short-term fasting in passerine birds during the postbreeding, molting and migratory period. Condor 99:113–122
Koffijberg K, Van Eerden MR (1995) Sexual dimorphism in the cormorant Phalacrocorax carbo sinensis: possible implications for differences in structural size. Ardea 83:37–46
Kortland A (1995) Patterns of pair-formation and nest-building in the European cormorant Phalacrocorax carbo sinensis. Ardea 83:11–25
Lessells CM, Krebs JR (1989) Age and breeding performance of European bee-eaters. Auk 106:375–382
Lill A, Yachou-Wos L, Johnstone CP (2013) Are haematocrit and haemoglobin concentration reliable indicators of body condition in adult Welcome Swallows? Emu 113:93–98
Liordos V, Goutner V (2008) Sex determination of Great Cormorants (Phalacrocorax carbo sinensis) using morphometric measurements. Waterbirds 31:203–210
Lozano GA, Perreault S, Lemon RE (1996) Age, arrival date and reproductive success of male American redstarts Setophaga ruticilla. J Avian Biol 27:164–170
Marzluff JM (1988) Do pinyon jays alter nest placement based on prior experience? Anim Behav 36:1–10
Minias P (2014) Evolution of within-colony distribution patterns of birds in response to habitat structure. Behav Ecol Sociobiol 68:851–859
Minias P (2015) The use of hemoglobin concentrations to assess physiological condition in birds: a review. Conserv Physiol 3:cov007. doi:10.1093/conphys/cov007
Minias P, Kaczmarek K (2013a) Concentrations of plasma metabolites as predictors of nestling condition in the Great Cormorant (Phalacrocorax carbo sinensis). Ornis Fenn 90:142–150
Minias P, Kaczmarek K (2013b) Is it always beneficial to breed in the centre? Trade-offs in nest site selection within the colony of a tree-nesting waterbird. J Ornithol 154:945–953
Minias P, Włodarczyk R, Janiszewski T (2015) Opposing selective pressures may act on the colony size in a waterbird species. Evol Ecol 29:283–297
Møller AP (1989) Nest site selection across field-woodland ecotones: the effect of nest predation. Oikos 56:240–246
Morrison ML, Slack RD, Shanley E Jr (1978) Age and foraging ability relationships of Olivaceous Cormorants. Wilson Bull 90:414–422
Nelson JB (2005) Pelicans, cormorants, and their relatives: the pelecaniformes. Oxford University Press, New York
Nisbet ICT, Dann P (2009) Reproductive performance of little penguins Eudyptula minor in relation to year, age, pair-bond duration, breeding date and individual quality. J Avian Biol 40:296–308
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Piper WH (1997) Social dominance in birds. Curr Ornithol 14:125–187
Post W (1990) Nest survival in a large ibis-heron colony during a three-year decline to extinction. Colon Waterbird 13:50–61
Pryke SR, Rollins LA (2012) Mothers adjust offspring sex to match the quality of the rearing environment. Proc R Soc Lond B 279:4051–4057
Pugasek BH (1993) Chick growth in the California gull: relationships with hatching asynchrony and parental age. Colon Waterbird 16:183–189
Pugasek BH, Diem KL (1983) A multivariate study of the relationship of parental age to reproductive success in California gulls. Ecology 64:829–839
Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P (2003) Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J Anim Ecol 72:765–776
Roberts G (1996) Why individual vigilance declines as group size increases. Anim Behav 51:1077–1086
Robertson RJ, Rendell WB (2001) A long-term study of reproductive performance in tree swallows—the influence of age and senescence on output. J Anim Ecol 70:1014–1031
Słomczyński R, Kaliński A, Wawrzyniak J, Bańbura M, Skwarska J, Zieliński P, Bańbura J (2006) Effects of experimental reduction in nest micro-parasite and macro-parasite loads on nestling hemoglobin level in blue tits Parus caeruleus. Acta Oecol 30:223–227
Thompsoan KR, Furness RW (1991) The influence of rainfall and nest-site quality in the population dynamics of the Manx shearwater Puffinus puffinus. J Zool 225:427–437
Velguth KE, Payton ME, Hoover JP (2010) Relationship of hemoglobin concentration to packed cell volume in avian blood samples. J Avian Med Surg 24:115–121
Weimerskirch H, Barbraud C, Lys P (2000) Sex differences in parental investment and chick growth in wandering albatrosses: fitness consequences. Ecology 81:309–318
Wojczulanis-Jakubas K, Minias P, Kaczmarek K, Janiszewski T (2013) Late-breeding great cormorants Phalacrocorax carbo sinensis produce fewer young of the more vulnerable sex. Ibis 155:626–631
Yorio P, Quintana F (1997) Predation by Kelp Gulls Larus dominicanus at a mixed-species colony of Royal Terns Sterna maxima and Cayenne Terns Sterna eurygnatha in Patagonia. Ibis 139:536–541
Acknowledgments
The study was performed under the permissions of the Bioethical Commission and the Regional Environmental Protection Directorate in Łódź, Poland. We are very grateful to all participants of the fieldwork. We thank two anonymous reviewers for helpful comments on the earlier draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Barbraud.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Janiszewski, T., Minias, P., Lesner, B. et al. Age effects on reproductive success, nest-site location, and offspring condition in the Great Cormorant Phalacrocorax carbo sinensis . J Ornithol 158, 193–202 (2017). https://doi.org/10.1007/s10336-016-1390-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1390-y