Abstract
An overlap in attributes of nest cavities used by Great Tit Parus major across Eurasia suggests similar nest site preferences within the geographical range, although the drivers of these preferences are unclear. To determine whether preferred cavities provide conditions enhancing successful reproduction, we investigated the breeding performance of Great Tits in relation to tree cavity characteristics using data collected during 2008–2011 in primeval conditions (Białowieża National Park, Poland). Here, tree cavities are diverse and superabundant but nesting birds are at risk from a variety of predators. According to expectations, nest losses were high (60 % of Great Tit nests failed), mostly due to predation (69 % of nest failures). The risk of nest failure varied with nest cavity attributes. Compared to successful nests, failures were situated higher above the ground and placed closer to the cavity entrance. Very deep cavities with narrow entrances and strong livings walls provided effective protection against larger predators (e.g., martens, woodpeckers), unable to enter the cavity or pull out the contents. Yet, such holes were no barrier for the smallest predators (e.g., Forest Dormouse Dryomys nitedula), which were able to enter any Great Tit nest cavity and destroyed most of the nests. Avoiding small predators would give a selective advantage to the birds, but this seems hardly possible to achieve. We conclude that tree cavities preferred by the tits show a combination of properties which are a compromise for avoiding predation (the strongest selective pressure) and providing the minimum requirements (sufficient nest illumination, microclimate, protection against nest soaking) for development and growth of young.
Zusammenfassung
Bruterfolg bei der Kohlmeise und ihr Zusammenhang mit Eigenschaften natürlicher Nisthöhlen in einem Urwald
Überschneidungen in den Eigenschaften von Nisthöhlen von Kohlmeisen (Parus major) in ganz Eurasien legen ähnliche Präferenzen für Nistplätze innerhalb dieses geographischen Gebiets nahe, wobei allerdings die Grundlagen für diese Präferenzen unklar sind. Um festzustellen, ob bevorzugte Höhlen Bedingungen bieten, die für die Fortpflanzung förderlich sind, untersuchten wir den Bruterfolg von Kohlmeisen im Zusammenhang mit Charakteristika der Baumhöhlen anhand von Daten, die zwischen 2008 und 2011 in einem Urwald aufgenommen wurden (Białowieża National Park, Polen). Hier sind Baumhöhlen divers und mehr als genug vorhanden, aber die nistenden Vögel stehen unter dem Druck verschiedenster Prädatoren. Entsprechend der Erwartungen waren die Nestverluste hoch (60 % der Nester gingen verloren), meistens aufgrund von Prädation (69 % der Nestverluste). Das Risiko für einen Nestverlust hing zusammen mit Eigenschaften der Nisthöhlen. Im Vergleich zu erfolgreichen Nestern, lagen verlorengegangene Nester höher über Grund und näher am Eingang der Höhle. Sehr tiefe Höhlen mit schmalen Eingängen und dicken Wänden stellten einen effektiven Schutz gegen größere Prädatoren (z.B. Marder, Spechte) dar, die dort nicht in der Lage waren, in die Höhle zu gelangen oder ihren Inhalt herauszuziehen. Allerdings stellten solche Eingänge kein Hindernis dar für die kleinsten Prädatoren (z.B. den Baumschläfer Dryomys nitedula), die in der Lage waren, in jedes beliebige Kohlmeisennest einzudringen, so dass die Zerstörung der meisten Nester zu ihren Lasten geht. Die kleinen Prädatoren zu meiden, würde für die Vögel einen Selektionsvorteil bedeuten, aber das scheint kaum erreichbar zu sein. Wir schließen daraus, dass die von Kohlmeisen bevorzugten Nisthöhlen eine Kombination von Eigenschaften aufweisen, die einen Kompromiss darstellen zwischen Vermeidung von Prädation (dem stärksten selektiven Druck) und der Bereitstellung der minimalen Voraussetzungen für Entwicklung und Wachstum der Jungvögel (ausreichendes Licht im Nest, Mikroklima, Schutz gegen Überschwemmung des Nests).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adaptations of animals can only be fully understood by making observations in the natural environments in which they have evolved (Baker 1938; Lack 1965; Wesołowski 1983; Tomiałojć et al. 1984). As such, studies of the evolution of the nest site preferences of cavity-nesting forest birds should, preferably, be conducted in the least transformed habitats (ideally, primeval woodlands), and concern birds using tree cavities rather than nest boxes (reviewed in Wesołowski 2011). In Europe, human exploitation of forests has continued for centuries. Widespread practices used in forest management, such as the removal of old, dying or dead trees and/or certain tree species containing a high number of cavities, has led to decreased availability of nest sites for cavity-nesting birds (Newton 1994). Additionally, human persecution has resulted in an impoverished predator fauna in many areas (Wesołowski 1983; Tomiałojć et al. 1984). Conditions near to primeval have been preserved in only a few places in Europe, including the strictly protected forest within the Białowieża National Park (hereafter BNP), in eastern Poland.
The primeval Białowieża forest gives a rare opportunity to observe the adaptations of birds living in habitats unaffected by extensive human interference (Tomiałojć and Wesołowski 2005). Here, tree cavities are diverse (varying in origin, location and size) and available in excess for non-excavating, cavity-nesting birds, which can choose their preferred nest sites (reviewed in Wesołowski 2007). Consequently, inter- and intraspecific competition for nest sites is unimportant and usurpation of nest holes by other species is very rare (e.g., Walankiewicz and Mitrus 1997; Wesołowski 2003). The forest supports a rich and diverse predator fauna of over 30 species, which depredate birds and/or their nests (Tomiałojć et al. 1984). The most important predators of cavity-nesting birds include the Great Spotted Woodpecker Dendrocopos major, Yellow-necked Mouse Apodemus flavicollis, Forest Dormouse Dryomys nitedula, Red Squirrel Sciurus vulgaris, Pine Marten Martes martes, and Weasel Mustela nivalis (Walankiewicz 1991, 2002; Wesołowski 2002; Wesołowski and Rowiński 2004, 2012; Czeszczewik et al. 2008). These predators have different modes of attack, and are variously active throughout the day and night, accessing all parts of the forest from the ground to the tree canopy. Therefore, they pose a serious threat for nesting birds and exert a strong selective pressure upon them to breed in safe places (reviewed in Wesołowski and Tomiałojć 2005).
As shown previously, knowledge of the breeding ecology of cavity nesters would be biased if based on nest box studies alone (e.g., van Balen et al. 1982; Robertson and Rendell 1990; Purcell et al. 1997; Czeszczewik et al. 1999; Mitrus 2003; Wesołowski 2011). Nest boxes provide conditions strikingly different from tree cavities for the birds nesting in them. Among others, they are of uniform dimensions, situated at a similar height (reviewed in Lambrechts et al. 2010), and constructed in a way to minimise predation and nest soaking—the main mortality factors of cavity nesters in natural situations (reviewed in Wesołowski 2011). In consequence, the nesting success of birds using nest boxes is usually very high, much higher than in tree cavities (e.g., Nilsson 1975; East and Perrins 1988; Purcell et al. 1997; Mitrus 2003). Despite the observed discrepancies in breeding productivity between birds using nest boxes and tree cavities, studies of European cavity nesters using natural nest sites are scant, even for an otherwise well-studied species, such as the Great Tit Parus major.
The Great Tit is a non-excavating, cavity nester, which inhabits a wide range of wooded habitats across Eurasia (Glutz von Blotzheim and Bauer 1993). It frequently uses nest boxes, to which it appears attracted, and nest box provision can often increase the breeding densities to artificially high levels that are unrecorded in natural situations (reviewed in Glutz von Blotzheim and Bauer 1993; Wesołowski 2011). Partly because of this reason, the Great Tit has become one of the most intensively studied bird species in Europe (1807 papers up to 2010, Lambrechts et al. 2010). Although the literature on Great Tit breeding ecology is vast (Glutz von Blotzheim and Bauer 1993), it is based almost exclusively on nest box observations. Studies of Great Tits breeding in tree cavities are very rare, with descriptions of natural nest sites coming from only a few papers (reviewed in Maziarz et al. 2015). According to these and a previous study in BNP (Maziarz et al. 2015), Great Tits most often occupy non-excavated, very deep and spacious cavities, with elongated and narrow openings, situated at intermediate heights in living tree trunks, which they appear to prefer. The results of Löhrl’s (1970, 1977, 1986) experiments with nest boxes of different dimensions, and situated at various heights above the ground, show a similar pattern of cavity occupation by Great Tits. This suggests similar nest site preferences of Great Tits across Eurasia, but the drivers of these preferences remain unclear. Presumably, the tree cavities most frequently used (preferred) by Great Tits would provide conditions favouring successful reproduction.
A relationship between the properties of nest cavities and breeding success has been shown for several hole-nesting passerines (e.g., Ludescher 1973; Nilsson 1984; Alatalo et al. 1990; Walankiewicz 1991; Albano 1992; Wesołowski 2002; Czeszczewik and Walankiewicz 2003; Wesołowski and Rowiński 2004, 2012; Broughton et al. 2011), but such information is currently almost lacking for the Great Tit (Nilsson 1984). As predation is the primary cause of mortality in many bird species (Nice 1957; Ricklefs 1969; Wesołowski and Tomiałojć 2005), it may also pose the main selective pressure on Great Tits, affecting nest site choice by the birds. Yet, scarce data for the causes and frequency of Great Tit nest losses in tree cavities (see Nilsson 1984; East and Perrins 1988; Deng and Gao 2005) prevent this from being ascertained.
Apart from predation, Great Tits can face other problems associated with using tree cavities that may affect the breeding success of birds, e.g., limited nest illumination, nest soaking, a cold and humid microclimate, and/or hindered ventilation (e.g., Howe and Kilgore 1987; East and Perrins 1988; Wesołowski et al. 2002; Wesołowski and Maziarz 2012; Maziarz and Wesołowski 2013, 2014). Minimising these problems could conflict with predator avoidance in the selection of a nest site by birds (Table 1), and force birds to compromise between the competing requirements in order to choose the best possible option for successful reproduction (e.g., Löhrl 1977, 1986; Wesołowski and Maziarz 2012; Maziarz and Wesołowski 2013, 2014).
In this study, we aimed to investigate the fate of Great Tit nests and causes of nest losses in natural cavities, using four years of data collected during an intensive study in BNP. We assumed that if the nest site choice by Great Tits was adaptive, the chances of successful reproduction would relate to cavity characteristics, and if predation pressure was the main selective factor shaping nest site selection by birds, it would be the main cause of Great Tit nest failures and predation rates would vary with the nest cavity properties (location and dimensions). We expected that the highest protection against predators would be provided by cavities with: (a) a small entrance, preventing entry by predators larger than the birds themselves; (b) great depth, allowing the nest to be placed beyond the reach of the paw or beak of a predator unable to enter the cavity; (c) hard walls of living wood, which are difficult to destroy by a predator that is unable to reach the nest by other means; (d) originating from processes of wood decay, being more difficult to access by predatory Great Spotted Woodpeckers than woodpecker-excavated holes; (e) greater height above the ground, hindering detection and access by ground foraging predators (Table 1; e.g., Nilsson 1984; Alatalo et al. 1990; Walankiewicz 1991; Tomiałojć 1993; Wesołowski 2002; Mitrus and Soćko 2008; Broughton et al. 2011). However, if selective forces other than predation were important, then birds would modify their nest site decision in order to meet the alternative requirements crucial for successful reproduction. This may include selection of appropriately shallow cavities with relatively large entrances, thereby providing sufficient nest illumination and/or ventilation, or cavities with dead walls to provide a relatively warm and dry microclimate with a low risk of nest soaking (Table 1). We discuss our results in the evolutionary context of nest site choice by Great Tits being a trade-off between safety and other selective forces.
Methods
Study area
The Białowieża Forest is a vast complex of c. 1500 km2, situated at the Polish-Belarusian border (co-ordinates of Białowieża 52°41′N and 23°52′E). Its western part, c. 45 % of the area, lies inside Poland. The forest represents a relic of the primeval temperate lowland forests of Europe. Although traces of human activity are known from the Neolithic period, intense timber-cutting did not start before the 20th century and barely afflicted the stands which are currently under strict protection within the BNP (area 47.5 km2). The preserved old growth stands are multi-storied, mixed-species and uneven-aged, with a canopy formed by trees over 200 years old (Tomiałojć and Wesołowski 2004). Standing dead timber and fallen trees are abundant (20–25 % of total wood volume; Bobiec 2002).
Most of the BNP consists of three distinct types of old-growth stands: oak-lime-hornbeam (Tilio-Carpinetum, 44 % of the BNP area), riverine ash-alder (Circaeo-Alnetum, Carici elongatae-Alnetum, 22 % of the BNP area) and coniferous (Peucedano–Pinetum, 28 % of the BNP area). Oak-lime-hornbeam stands are the richest in tree species and structurally the most diverse habitat. They are composed mostly of common hornbeams Carpinus betulus, small-leaved limes Tilia cordata, Pedunculate oaks Quercus robur, Norway spruces Picea abies, and Norway maples Acer platanoides, with small amounts of common ash Fraxinus excelsior, elms Ulmus spp., birches Betula spp. and aspens Populus tremula. For detailed descriptions, see, e.g., Tomiałojć et al. (1984) and/or Wesołowski et al. (2006, 2010).
Breeding data
Data on breeding Great Tits were collected in 2008–2011 in oak-lime-hornbeam stands, where breeding densities of this species are the highest in the BNP (up to 5 pairs/10 ha, Wesołowski et al. 2006, 2010). Observations were made in two plots: C (48 ha) and M (54 ha), which were permanently marked and situated 3 km apart (for detailed description of plots see Tomiałojć et al. 1984; Wesołowski et al. 2006, 2010). Nest boxes or artificial food were not provided.
In order to find all nests on the study plots, adult Great Tits were intensively followed from before nest building began (beginning of April), during daily visits to the study plots. To establish the number of breeding pairs and nests to be found, prior to nesting, the birds were caught in nets with use of a dummy and playback, and individually marked with a combination of one numbered metal ring and three colour rings (details in Maziarz et al. 2015). During the nest searches, the birds’ movements were noted on field maps with special attention given to females carrying nest material to tree cavities.
Progression and fate of nests was monitored on regular visits, mostly from the ground. After incubation had commenced, the cavities were inspected to determine clutch size. The nest contents were checked with an illuminated small mirror, those in lower-situated cavities from the ground or a ladder, and higher ones by climbing. The cavities were inspected again around the time when the young hatched to establish hatching date based on their age according to Winkel’s (1970) developmental criteria. In a small number of inaccessible cavities, the hatching date was determined during visits repeated every second day around the anticipated day of hatching; the date before the first day on which adult behaviour indicated that nestlings were present (the female returned with food, or the male entered the cavity with food when the female was outside) was taken as the hatching date. In accessible cavities, nestlings were counted within 4 days of hatching.
The fledging date was estimated by adding 18 days to the recorded hatching date (Glutz von Blotzheim and Bauer 1993; Wesołowski et al. unpubl. data). The number of fledglings was taken as the nestling count on the last day when it was safe to extract them from the cavity, i.e., when 13–14 days old, as after that age, the risk of premature fledging would be too high. The nestlings were ringed, but this manipulation did not affect young mortality, as the percentage of nest losses in cavities with ringed young was lower (13 % lost of 67 nests) than in cavities with un-ringed nestlings (28 % lost of 18 nests). Around the estimated time of fledging, cavities were observed daily from a distance until no adults were recorded bringing food. Nests were considered successful if 18-days-old young were still present in the nest and no signs of attempted predation (see below) were detectable the following day. If there was no activity at the hole when young were about to fledge (17–18 days old), then searches were made for parents collecting and carrying food to fledged young. If the family was found, the nest was treated as successful. Nests were considered to have failed if there was no progress in nesting, and/or eggs or young disappeared or parents were absent for a minimum 90-min period on at least two consecutive observations before the estimated fledging date.
In the case of nest failures in accessible cavities (161 of 181 cavities), the nest contents, cavity entrance, the trunk around and below the entrance, and the ground surface around the nest tree were carefully examined for signs of predation and the remains of eggs, young, and/or adults. Based on detailed descriptions of the nest and its vicinity taken at the cavity, causes of nest failure were classified as: (a) burial—intact eggs or nestlings covered by wood debris; (b) ‘desertion’ by parents—no signs of predation, eggs or dead nestlings in intact nest; (c) entrance blocked—spruce cones wedged in cavity by (presumably) a woodpecker; (d) flooding—nest material soaked, wet intact eggs or nestlings; (e) predation—empty or slightly tousled nest cup, remnants of eggs, nestlings or adults, the predator inside or identified by faeces or appearance of the nest (Nowakowski and Boratyński 2001); (f) unknown—the nest situated in an inaccessible cavity, or identifying the cause of nest loss was impossible.
Nest cavity description
The following characteristics of Great Tit nest cavities were recorded: (a) tree species; (b) trunk girth at breast height; (c) hole height above the ground (estimated from the ground for cavities up to 10 m, otherwise measured to nearest 1 m using a clinometer); (d) hole origin—woodpecker-excavated or non-excavated; (e) location on the tree—trunk or limb/branch; (f) cavity walls of live or dead wood; (g) entrance shape—elongated/slit, rounded in a knothole (conical bulge formed at site of a previous limb break) or irregular; (h) entrance inclination (upward, vertical or downward).
Nest cavity measurements were taken directly after the young had fledged or the nest had failed, using a collapsible ruler and a flexible torch. Measurements included: (a) entrance diameter—smallest and greatest dimension of the cavity opening cross-section, taken in a vertical or horizontal plane; (b) cavity diameter—smallest and greatest dimension of the cavity’s interior horizontal cross-section taken at the level of the top of the nest material; (c) nest distance from the entrance—vertical distance between the lower edge of the entrance and the top of the nest material; (d) ‘safety’ distance—distance between the bottom edge of the external wall of the entrance and the centre of the nest at its rim level, indicating how far a predator would have to reach to remove eggs or nestlings.
The degree of isolation of tree crowns was assessed in 2009 for all Great Tit nest trees containing first breeding attempts during 2008–2009. This was expressed as the percentage of the crown that did not abut or intersect neighbouring trees, and was estimated ‘by eye’ to the nearest 5 % by two independent observers. The observers always yielded similar scores (within ±10 %).
Data analysis
As recording all breeding parameters and/or cavity measurements was not always possible for each nest, sample sizes differed between analyses. Cavities used in more than 1 year were treated as independent in analyses, as they were always used by different birds between years (exercising independent cavity selection), and cavity dimensions changed over time due to tree growth/decay, which limited potential pseudo-replication (Maziarz et al. 2015). Excluding such ‘reused cavities’ from analyses would bias results of nest site decisions towards only those Great Tit pairs which used unique (individual) cavities, giving incomplete information. Only the first breeding attempts were included in the analyses, as following the birds and finding all the repeated and second clutches was hardly possible after the tree leaves had developed. Every year, almost all first breeding attempts were found (90–100 %) in plots C and M, mainly at the stage of nest building and egg laying (87 % of all first breeding attempts found), therefore the nest losses were expressed as a percentage of all first breeding attempts and the use of Mayfield’s (1961) method of estimating failure rates was considered unnecessary. Partial clutch losses were defined as the difference between the clutch size and the number of young hatched, and partial brood losses as the difference between the number of hatched young and the number of fledglings.
Cavity floor and entrance area were calculated as 0.5 (smallest diameter) × 0.5 (greatest diameter) × 3.14, assuming the geometric form of an ellipse.
For statistical calculations, we used non-parametric tests following formulae in Statistica 10.0 (StatSoft Inc. 2011), and all probabilities were two-tailed.
Results
More than half of the Great Tit pairs (60 % of 181), which commenced first breeding attempts, failed to reproduce. Causes of total nest failure were determined for 92 of 109 lost attempts. Fifteen of the remaining 17 unsuccessful nests, where the cause was unknown (Table 2), were located in snags, dead limbs or very large trees, where climbing to inspect the nest was not considered safe.
Predation was the principle cause of nest losses, accounting for 69 % of 109 failures. Other causes were desertion (12 cases), nest burial under wood debris (two), flooding (two), and entrance blockage with spruce cones, probably inserted by a Great Spotted Woodpecker (one case; Table 2). The frequency of nest failure was similar for nests with eggs and nestlings (Table 2). Remains of adult birds were found in 17 of 75 depredated nests, mostly during the egg stage (12 of 35 nests were depredated during egg laying or incubation), and less often in the nestling period (5 of 40 nests robbed during or after hatching). In all cases, the victims were presumably incubating or brooding females (only female Great Tits incubate/brood young).
The risk of nest failure depended on nest cavity location. Nesting in trunks of trees (56 % lost of 136 nests) was usually safer than in limb cavities (76 % of 42; Fisher’s exact test, P = 0.020). Nest losses also tended to be less frequent in cavities with slit-shaped entrances (51 % of 81) than with other entrance shapes (67 % of 67; Fisher’s exact test, P = 0.046). The proportion of nest failures was 15–19 % higher in woodpecker-excavated cavities (seven of nine nests lost) or holes in dead wood (nine of twelve nests lost), compared to non-excavated (59 % of 170) or live wood cavities (60 % of 166), though the differences were not statistically significant (Fisher’s exact test, P > 0.3). Cavities with upward-facing entrances (n = 55 nests) contained 53 % losses, only slightly less than the tree cavities with sideways or downward-facing entrances (63 % of 104 nests in total; Fisher’s exact test, P = 0.24). Failure rates were comparable in limes (54 % of 24 nests), hornbeams (58 % of 119) and ‘other’ tree species (64 % of 22), but highest in maples (81 % of 16 nests; for all tree species: χ 2 = 3.7, df = 3, P = 0.30). Among other tree species, four of seven nests were lost in ash trees, all six were lost in oaks, two of four in aspens, one of four in spruces, and the only nest in birch also failed.
The proportion of nest failures increased with the hole height above the ground; at heights of 10–20 m, where the canopy layer was most developed (Tomiałojć and Wesołowski 1990), nest failures were 4–5 times higher than the proportion of successful nests (Fig. 1). Consequently, the mean height of successful nests was significantly lower than that of depredated nests (Table 3).
The crowns of the nest trees used by Great Tits in 2008–2009 were a median 50 % (n = 84) isolated from neighbouring trees, which was similar for successful and failed nests (respective medians 50 and 45 %, n = 32 and 52). However, among trees with nest holes situated ≥10 m high, isolation tended to be greater for successful nests (median 68 %, n = 10) than failed ones (median 50 %, n = 26), but the difference was not significant (Mann–Whitney test, Z adjusted = 1.6, P = 0.11). Among the remaining, lower situated holes, the tree crowns were a median 23 % isolated for successful attempts (n = 22) and 30 % for lost attempts (n = 26; Mann–Whitney test, Z adjusted = 0.69, P = 0.49).
Compared to depredated nests, successful broods were recorded in cavities with only a slightly narrower entrance (Table 3). However, in successful cavities, the median entrance area was 8 cm2 larger and the nest and ‘safety’ distances were respectively 6 cm and 4 cm, greater than in depredated cavities (Table 3). The floor area did not differ between successful and depredated nests (Table 3).
According to the size of the cavity entrance passable by different predators, given in Wesołowski (2002), all Great Tit nest cavities were accessible to smaller predators, such as Forest Dormice, weasels and Yellow-necked Mice, which were able to enter through any openings passable by the birds (i.e., with the smallest entrance diameter of 2 cm; Maziarz et al. 2015). Additionally, more than half of Great Tit nest cavities (57 % of 148) had a smallest entrance diameter of ≥3.2 cm, permitting entry by medium-sized predators, such as Red Squirrels. However, only 20 % of nest holes had entrances large enough (≥4.5 cm) to allow entry to larger predators, such as Great Spotted Woodpeckers or Pine Martens.
In cavities where the opening was too small for the predator to enter fully, the nest could still be reached with its paw or beak if placed close enough to the opening (within c. 16 cm; see review in Wesołowski 2002). In cavities with ‘safety’ distances ≥20 cm, the nests could be beyond reach of all predators unable to enter the hole. In BNP, only 3.5 % of 143 Great Tit nest cavities had a ‘safety’ distance less than 16 cm, while for 79 % it was more than 20 cm (Fig. 2). Nesting very close to the cavity entrance was indeed risky for Great Tits; of five cavities with a ‘safety’ distance <16 cm, four were depredated, although the proportion of depredated nests increased with decreasing ‘safety’ distance also among those placed further than this distance (Fig. 2). Damage to cavity walls, indicating predation by Great Spotted Woodpeckers (Perrins 1979), or claw marks and the nest pulled out through the cavity entrance, indicating Pine Martens (Walankiewicz 2002; Misík and Paclík 2007), were not recorded.
Percentage of depredated Great Tit Parus major nests in relation to the ‘safety’ distance (cm; the nest distance from the external wall of the cavity entrance, showing how far the predator would have to push in its paw or beak to reach the nest). Sample size for each category is given above the x-axis. The proportion of depredated nests decreased significantly with an increasing ‘safety’ distance (χ 2 = 472.7, df = 5, P < 0.001)
The Forest Dormouse was the most important nest predator in the current study, responsible for 51 % of 75 depredated nests. Individual Forest Dormice were found in seven Great Tit nest cavities, and for others, the presence of characteristic faeces, hair and/or the pattern of brood damage allowed identification (see Nowakowski and Boratyński 2001). In 12 of the 17 nests containing remnants of adult Great Tits, Forest Dormouse was the predator. For most other depredated nests, predator identification was usually impossible, but intact or disturbed nests (empty or containing bird remains) suggested that the predator was able to enter the cavity (28 of 75 cases). In the next seven cavities, chewed young or their feathers were found, indicating predation by mustelids, and at one nest cavity, a Pygmy Owl Glaucidium passerinum was recorded attacking and killing an adult Great Tit bringing food to the nest (the nestlings disappeared by the next day, presumably taken by the owl). In another cavity, the nest was destroyed and eggs were smashed.
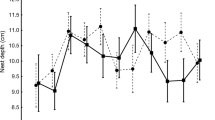
Most nests destroyed by a Forest Dormouse were in non-excavated cavities (89 % of 37) in tree trunks (73 %), mainly in ‘knotholes’ (52 % of 27) or cavities with slit-like openings (33 %). More than half were in hornbeams (55 % of 38), with 26 % in maples. Dormouse-depredated nests were situated a median 10 m (4.5–23 m, n = 38) above the ground, almost twice as high as successful nests (median 6 m, 0.5–27 m; Table 3; Mann–Whitney test, Z adjusted = 3.9, P < 0.001). They were also placed in trees thicker (median girth at breast height 165 cm, n = 38) than those harboring successful nests (median girth 151 cm, n = 69; Mann–Whitney test, Z adjusted = 2.2, P = 0.03). In all of these cases, the Forest Dormouse entered the hole without damaging it. The smallest entrance diameter in such cavities was 2.0–6.5 cm (median 3.5 cm, n = 34), only slightly larger than cavities with successful nests (median 3.2 cm, 2–14.4 cm; Table 3; Mann–Whitney test, Z adjusted = 1.3, P = 0.21). The nests destroyed by dormice were usually situated much closer to the entrance (medians: nest distance = 16 cm, ‘safety’ distance = 23 cm) than successful nests (medians: 23 and 27 cm, respectively; Mann–Whitney test, Z adjusted = 2.5–2.9, P < 0.012). Consequently, most of the nests in maple cavities (10 of 16), which were usually situated higher above the ground and had shorter ‘safety’ distances than hornbeam cavities (Fig. 3), were destroyed by this predator.
Distribution of ‘safety’ distances (the nest distance from the external wall of the cavity entrance, showing how far the predator would have to push in its paw or beak to reach the nest) in Great Tit Parus major nest cavities plundered by Forest Dormouse Dryomys nitedula, in relation to the hole height above the ground. Nests located in hornbeams (white diamonds) and maples (black triangles) are shown. For other tree species (six nests), the respective median (and range) for hole height and ‘safety’ distance, respectively, was 13 m (7–17) and 23 cm (14–35)
The brood productivity, in general, depended only slightly on the cavity floor area. This was most pronounced for the clutch size (7–13 eggs, median 10 eggs), which increased with the cavity floor area (r s = 0.27, P = 0.004, n = 111; Fig. 4). There was no such relationship for the number of young hatched or the number of fledglings (r s = 0.21 and 0.03, P > 0.08, n = 69 and 51, respectively). Neither partial clutch losses nor partial brood losses varied with cavity floor area (r s = −0.02 and 0.12, P > 0.4, n = 67 and 41, respectively).
Discussion
Great Tits in the BNP’s primeval forest suffered one of the highest rates of nest loss (60 %) recorded for this species breeding in tree cavities (22 % losses in Sweden to 72 % in Netherlands and Spain; reviewed in Maziarz 2012), though limited and incomplete data from other areas prevent detailed comparisons. For nest box populations of Great Tits, overall nest losses (with a nest as a unit of measurement) are rarely mentioned in the literature, but are usually much lower than in tree cavities (18–43 % for first breeding attempts; Gibb 1950; reviewed in Orell and Ojanen 1983; East and Perrins 1988).
In BNP, predators were responsible for most Great Tit nest losses (69 %). Predation of adults at the nest hole when feeding young was observed only once (the bird being caught by a Pygmy Owl), but could be more frequent, and, so, explaining some cases of apparent nest desertion, which was the second most common cause of nest failure. Nest soaking was rarely recorded during 2008–2011, although prolonged, intensive rainfall did not occur during the study period, and some cases may also have gone undetected if the nest dried out before inspection, leaving no signs in an abandoned nest.
The causes of Great Tit nest losses in tree cavities are mostly unknown from other areas, which impedes comparisons and underlines the need for more studies on birds nesting in natural sites. As in BNP, Nilsson (1984) found that predators caused most (62 %) of Great Tit nest failures in tree cavities in Sweden. In China, however, predation accounted for only 16 % of failures, less than for cavity usurpation by other bird species (63 %), although this included nests where egg laying had not commenced (Deng and Gao 2005). In England, nest soaking was responsible for frequent nest desertion, and was the main cause of nest failures in one year, but other causes were unknown (East and Perrins 1988).
Predation, by being the main cause of Great Tit nest failures in BNP, appears to be the main selective force for breeding birds; its avoidance can most enhance the chances of successful reproduction. As well as destroying the nests, predators constituted a serious threat for adult Great Tits by killing them in nests during the incubation and early nestling stage (adult remains, probably mostly females, were recorded in 23 % of depredated nests). As an active nest defence, such as the ‘hissing’ display, aimed at threatening a predator, given by birds disturbed within the cavity (Glutz von Blotzheim and Bauer 1993; Krams et al. 2014), can be used only as a last means of defence (Edmunds 1974), selection of a safe nest location (made mainly by females; Perrins 1979) could be primary in predator avoidance. Therefore, in order to reproduce successfully, the birds should first focus on finding a secure cavity for nesting.
In BNP, Great Tits have free access to diverse and superabundant tree cavities from which they can choose the most preferable ones (Maziarz et al. 2015). Because the proportion of nest failures (mostly due to predation) was related to the cavity properties (situation and dimensions), as shown in this study, the birds were able to diminish the risk of predation by occupying tree cavities with specific properties enhancing nest safety. This emphasises an adaptive value of nest site choice by Great Tits.
The initial expectation of nests located higher above the ground being safer (Table 1; e.g., Nilsson 1984; Albano 1992; Tomiałojć 1993; Mitrus and Soćko 2008; Broughton et al. 2011), was not met in this study and needed revision. This was because of more frequent predation by Forest Dormice in higher-situated holes. Nilsson (1984) found no relationship between nest losses and the height of Great Tit nest cavities, although Wesołowski and Rowiński (2012) recorded a similar pattern to our results in Blue Tit Cyanistes caeruleus. The predation pressure observed in BNP was especially pronounced for Great Tit nests situated ≥10 m above the ground, where most (c. 80 %) failed. As crown isolation tended to be lower for trees with failed nests situated at heights of ≥10 m, intersecting tree crowns probably aided movements of arboreal Forest Dormice and improved their ability to find these high nests. The most developed part of the tree canopy layer at heights of 10–20 m (Tomiałojć and Wesołowski 1990), could further facilitate the detection of nests by arboreal predators. Presumably, the birds might better avoid predation by Forest Dormice by nesting at lower heights, but this may have incurred other costs, such as a colder microclimate (Maziarz and Wesołowski 2013) or higher risk of nest soaking due to stem flow and sap drain from interior cavity walls (Wesołowski et al. 2002). Thus, nesting at intermediate heights, as most frequently observed in BNP (Maziarz et al. 2015), could reflect a trade-off between these counteracting pressures.
Although we found insignificant differences in nest losses between woodpecker-excavated and non-excavated cavities, or those in dead wood or live wood, Great Tit avoidance of excavated cavities with dead walls may have a biological underpinning (Maziarz et al. 2015). Most of the Great Tits (80–90 %) in BNP nested in cavities with entrances too small for larger predators to enter, such as Great Spotted Woodpeckers or Pine Martens, or which prevented them from reaching the nest from outside (‘safety’ distance ≥20 cm, reviewed in Wesołowski 2002). In order to gain access, the predators would have to destroy the cavity wall, but this was largely precluded by the hard and thick walls of the great majority of nest cavities, which were non-excavated (94 %), situated in living trees (95 %), and typically in hard-wooded hornbeams (c. 70 %; Maziarz et al. 2015). In consequence, Great Tit nests were mostly inaccessible to larger predators, which destroyed them sporadically. This type of predation was not recorded among first breeding attempts during our study period, and has only occasionally been documented for the Great Tit in BNP. This involved three cases of Pine Marten predation on repeated or second breeding attempts in 2008–2011, and ten records of nests pulled out in 1976–2007, along with woodpecker excavation of the cavity on five occasions (Wesołowski et al. unpubl.). Nilsson’s (1984) results—no predation by Great Spotted Woodpeckers of Great Tit nests in tree cavities (which had similar properties to those in BNP; Maziarz et al. 2015), but frequent predation of nests in nest boxes, which are easier for a woodpecker to penetrate—lead to a similar conclusion to our findings. Thus, probably because of the higher security, Great Tits preferred cavities in living wood despite some costs associated with using them: a humid and rather cold microclimate, which may commit female Great Tits to greater energy expenditure when incubating eggs and brooding young (reviewed in Maziarz and Wesołowski 2013), or higher risk of nest flooding (Wesołowski et al. 2002).
Nesting in cavities with smaller, but not the smallest passable, entrances diverged from the assumptions of the secure cavity (Table 1), which probably reflected a degree of compromise by adults in meeting the minimum requirements of other constrains necessary for successful reproduction. Although the entrance size of 43 % of Great Tit cavities was small enough to block access to medium sized predators (such as Red Squirrel), it was not a barrier for the smallest predators (e.g., Weasel, Yellow-necked Mouse, or Forest Dormouse). To escape the smallest predators, Great Tits would have to nest in cavities with openings ≤1.5 cm wide (Wesołowski 2002), but would then be unable to enter the cavity themselves. Great Tits could possibly limit entry to medium-sized predators by using cavities with the smallest passable entrance (c. 2 cm diameter) more frequently, but then the light conditions inside might be too low for the birds to operate (Wesołowski and Maziarz 2012). In addition, deep cavities with very small entrances could have insufficient ventilation (Howe and Kilgore 1987). Then, the birds probably would have to nest closer to the entrance to maintain sufficient nest illumination and avoid hypoxia, placing them at greater risk from larger predators. As the smallest cavity entrance diameter was similar for successful and failed nests, nesting in cavities with the smallest passable openings brought little or no additional selective advantage for Great Tits. Thus, by occupying cavities with entrances slightly wider than the birds’ body size required, and with a slit shape (with larger area; Maziarz et al. 2015), Great Tits could balance predator avoidance and physical constraints of the cavity. This permitted them nesting at deeper, safer distances from the opening, and at an adequate illumination (Wesołowski and Maziarz 2012) or ventilation.
Contrary to the expectations of the nest security hypothesis (Table 1), the risk of nest failure did not depend on the cavity floor area, suggesting low selective pressure against using small cavities. Although less spacious cavities would probably help to reduce heat loss from the nest, the birds mainly selected larger cavities (Maziarz et al. 2015). Such behaviour could be explained by the benefits of using holes with a larger floor area, including the possibility of: building bulky nests in deep and spacious cavities that are better at insulating eggs and nestlings and protecting against nest soaking, the laying of larger clutches, and the avoidance of hyperthermia by allowing young to disperse in the cavity on hot days (Löhrl 1973).
The decreased predation with increasing nest distance from the cavity opening that we observed in BNP agreed with the assumptions of the secure nest cavity (Table 1). However, placing the nest much beyond the reach of larger predators (i.e., more than 20 cm from the entrance; Wesołowski 2002) could be expected to be superfluous and incur additional costs, such as insufficient nest illumination (Wesołowski and Maziarz 2012). Despite this, Great Tits in BNP have been shown to nest at a median ‘safety’ distance of 24 cm (Maziarz et al. 2015), which was associated with increased breeding success due to less predation also by small mammals. Although smaller predators were physically able to access these deep nest cavities (e.g., Forest Dormice depredated nests with ‘safety’ distances up to 46 cm), they did so less often than in shallower cavities where the nests were probably easier to detect. A selective advantage of using cavities with a greater than expected ‘safety’ distance was also found for Blue Tits and Pied Flycatchers Ficedula hypoleuca in BNP (Czeszczewik and Walankiewicz 2003; Wesołowski and Rowiński 2012), but not for the Marsh Tit Poecile palustris or the Nuthatch Sitta europaea which breed earlier and rarely suffer dormouse predation (Wesołowski 2002; Wesołowski and Rowiński 2004; Wesołowski and Cholewa 2009). Thus, the tendency to nest in much deeper cavities may be an anti-predator strategy for species whose breeding season more closely coincides with Forest Dormouse activity later in the spring (Juškaitis 2006; Adamík and Král 2008).
Although the Forest Dormouse was the main predator of Great Tit nests in BNP, responsible for half of all depredated nests, and escaping such predation would give a great selective advantage, it seems hardly possible for birds to achieve. While using very deep cavities situated at lower elevations could diminish the risk to some extent, breeding early in the spring, when Forest Dormice are still hibernating (Juškaitis 2006), probably could be the only means for Great Tits to avoid predation by this species completely. However, the birds did not take this opportunity, probably due to constraints on advancement of the breeding season. Despite being under strong predation pressure, the birds appear unable to further improve their chances of predator avoidance and successful breeding through nest site choice, indicating that Great Tits have reached their adaptive limits.
In summary, this is the first detailed study of the reproductive consequences of nest site choice by Great Tits under natural conditions. Avoiding predation (the main selective force) by breeding in safer cavities has the greatest influence on the birds’ breeding success, while other factors (e.g., nest soaking, cold and humid microclimate, insufficient nest illumination) important in extreme situations, can modify the birds’ decisions. The cavities most often used (preferred) by Great Tits are a compromise between opposing selective pressures that provide the greatest possible security and offer other conditions sufficient for the successful rearing of young. Very deep cavities with narrow entrances and strong walls of living wood give effective protection against larger predators. However, the nest cavities are not a barrier for the smallest predators able to enter any nest hole accessible to Great Tits. The Forest Dormouse poses the greatest threat for nesting Great Tits in BNP, accounting for half of all depredated nests, sometimes killing adults within. Although escaping Forest Dormouse predation would give a great selective advantage, it seems hardly possible for birds to achieve. We conclude that Great Tits have reached their adaptive limits in nest site selection, as changes in any direction would probably decrease the chances of successful reproduction.
References
Adamík P, Král M (2008) Nest losses of cavity nesting birds caused by dormice (Gliridae, Rodentia). Acta Theriol 53:185–192
Alatalo R, Carlson A, Lundberg A (1990) Polygyny and breeding success of Pied Flycatchers nesting in natural cavities. In: Blondel J, Gosler A, Lebreton JD, McCleery R (eds) Population biology of passerine birds. Springer, Berlin, pp 323–330
Albano DJ (1992) Nesting mortality of Carolina Chickadees breeding in natural cavities. Condor 94:371–382
Baker JR (1938) The evolution of breeding seasons. In: de Beer GR (ed) Evolution: essays on aspects of evolutionary biology. Clarendon Press, Oxford, pp 161–177
Bobiec A (2002) Living stands and dead wood in the Białowieża forest: suggestions for restoration management. For Ecol Manag 165:125–140
Broughton RK, Hill RA, Bellamy PE, Hinsley SA (2011) Nest-sites, breeding failure, and causes of non-breeding in a population of British Marsh Tits Poecile palustris. Bird Study 58:229–237
Czeszczewik D, Walankiewicz W (2003) Natural nest sites of the Pied Flycatcher Ficedula hypoleuca in a primeval forest. Ardea 91:221–230
Czeszczewik D, Walankiewicz W, Mitrus C, Nowakowski W (1999) Nest-box data of Pied Flycatcher Ficedula hypoleuca may lead to erroneous generalizations. Vogelwelt 120:361–365
Czeszczewik D, Walankiewicz W, Stańska M (2008) Small mammals in nests of cavity-nesting birds: why should ornithologists study rodents? Can J Zool 86:286–293
Deng WH, Gao W (2005) Edge effects on nesting success of cavity-nesting birds in fragmented Forest. Biol Conserv 126:363–370
East ML, Perrins CM (1988) The effect of nestboxes on breeding populations of birds in broadleaved temperate woodlands. Ibis 130:393–401
Edmunds M (1974) Defence in animals. Longman, New York
Gibb J (1950) The breeding biology of the great and blue titmice. Ibis 92:507–539
Glutz von Blotzheim U, Bauer KM (1993) Handbuch der Vögel Mitteleuropas, vol 13. Aula, Wiesbaden
Howe S, Kilgore DL Jr (1987) Convective and diffusive gas exchange in nest cavities of the Northern Flicker (Colaptes auratus). Physiol Zool 60:707–712
Juškaitis R (2006) Interactions between dormice (Gliridae) and hole-nesting birds in nestboxes. Folia Zool 55:225–236
Krams I, Vrublevska J, Koosa K, Krama T, Mierauskas P, Rantala MJ, Tilgar V (2014) Hissing calls improve survival in incubating female great tits (Parus major). Acta Ethol 17:83–88
Lack D (1965) Evolutionary ecology. J Appl Ecol 2:247–255
Lambrechts MM, Adriaensen F, Ardia DR et al (2010) The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol 45:1–26
Löhrl H (1970) Unterschiedliche Bruthöhlenansprüche von Meisenarten und Kleibern als Beitrag zum Nischenproblem. Verh dt zool Ges 64:314–317
Löhrl H (1973) Influence of nest-box size on clutch size of the great tit (Parus major). J Ornithol 114:339–347 (in German)
Löhrl H (1977) Ecological and ethological adaptations in hole-nesting birds. Vogelwarte 29:92–101 (in German)
Löhrl H (1986) Experiments on nest site selection in great tit, Parus major. J Ornithol 127:51–59 (in German)
Ludescher FB (1973) The marsh tit (Parus palustris) and the willow tit (Parus montanus) as sympatric sibling-species. J Ornithol 114:3–56 (in German)
Mayfield H (1961) Nesting success calculated from exposure. Wilson Bull 73:255–261
Maziarz M (2012) The nest sites’ characteristic and breeding success of great tit Parus major in primeval conditions (Białowieża National Park). PhD thesis, University of Wrocław (in Polish, English summary)
Maziarz M, Wesołowski T (2013) Microclimate of tree cavities used by Great Tits (Parus major) in a primeval forest. Avian Biol Res 6:47–56
Maziarz M, Wesołowski T (2014) Does darkness limit the use of tree cavities for nesting by birds? J Ornithol 155:793–799
Maziarz M, Wesołowski T, Hebda G, Cholewa M (2015) Natural nest sites of Great Tits (Parus major) in a primeval temperate forest (Białowieża National Park, Poland). J Ornithol 156:613–623
Misík J, Paclík M (2007) Predation on great spotted woodpecker (Dendrocopos major) nests by marten (Martes sp.). Sylvia 43:173–178
Mitrus C (2003) A comparison of the breeding ecology of Collared Flycatchers nesting in boxes and natural cavities. J Field Ornithol 74:293–299
Mitrus C, Soćko B (2008) Breeding success and nest site characteristics of Red-breasted Flycatchers Ficedula parva in a primeval forest. Bird Study 55:203–208
Newton I (1994) The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biol Conserv 70:265–276
Nice MM (1957) Nesting success in altricial birds. Auk 74:305–321
Nilsson SG (1975) Clutch size and breeding success of birds in nest boxes and natural cavities. Vår Fågelvärld 34:207–211
Nilsson SG (1984) The evolution of nest site selection among hole-nesting birds: the importance of nest predation and competition. Ornis Scand 15:167–175
Nowakowski WK, Boratyński P (2001) On identification of predation traces in breeding boxes. Not Ornitol 41:55–69 (in Polish, English summary)
Orell M, Ojanen M (1983) Breeding success and population dynamics in a northern Great Tit Parus major population. Ann Zool Fenn 20:77–98
Perrins CM (1979) British tits. Collins, London
Purcell KL, Verner J, Oring LW (1997) A comparison of the breeding ecology of birds nesting in boxes and tree cavities. Auk 114:646–656
Ricklefs RE (1969) An analysis of nesting mortality in birds. Smithson Contrib Zool 9:1–48
Robertson RJ, Rendell WB (1990) A comparison of the breeding ecology of a secondary cavity nesting bird, the Tree Swallow (Tachycineta bicolor), in nest boxes and natural cavities. Can J Zool 68:1046–1052
StatSoft Inc (2011) STATISTICA (data analysis software system), version 10. www.statsoft.com
Tomiałojć T (1993) Breeding ecology of the Blackbird Turdus merula studied in the primaeval forest of Białowieża (Poland). Part I. Breeding numbers, distribution and nest sites. Acta Ornithol 27:131–157
Tomiałojć L, Wesołowski T (1990) Bird communities of the primaeval temperate forest of Białowieża, Poland. In: Keast A, Blondell J, Helle P, Kikkawa J, Recher HW, Holmes RT (eds) Biogeography and ecology of forest bird communities. SPB Academic Publ bv, Hague, pp 141–165
Tomiałojć L, Wesołowski T (2004) Diversity of the Białowieża Forest avifauna in space and time. J Ornithol 145:81–92
Tomiałojć L, Wesołowski T (2005) The avifauna of the Białowieża Forest: a window into the past. Br Birds 98:174–193
Tomiałojć L, Wesołowski T, Walankiewicz W (1984) Breeding bird community of a primaeval temperate forest (Białowieża National Park, Poland). Acta Ornithol 20:241–310
van Balen JH, Booy CJH, van Franeker JA, Osieck ER (1982) Studies on hole-nesting birds in natural nest sites. 1. Availability and occupation of natural nest sites. Ardea 70:1–24
Walankiewicz W (1991) Do secondary cavity-nesting birds suffer more from competition for cavities or from predation in a primeval deciduous forest? Nat Area J 11:203–212
Walankiewicz W (2002) Breeding losses in the Collared Flycatcher Ficedula albicollis caused by nest predators in the Białowieża National Park (Poland). Acta Ornithol 37:21–26
Walankiewicz W, Mitrus C (1997) How nest-box data have led to erroneous generalizations: the case of the competition between Great Tit Parus major and Ficedula flycatchers. Acta Ornithol 32:209–212
Wesołowski T (1983) The breeding ecology and behaviour of Wrens Troglodytes troglodytes living under primaeval and secondary conditions. Ibis 125:499–515
Wesołowski T (2002) Anti-predator adaptations in nesting Marsh Tits Parus palustris: the role of nest site security. Ibis 144:593–601
Wesołowski T (2003) Bird community dynamics in a primaeval forest—is interspecific competition important? Ornis Hung 12–13:51–62
Wesołowski T (2007) Lessons from long-term hole-nester studies in a primeval temperate forest. J Ornithol 148(Suppl 2):S395–S405
Wesołowski T (2011) Reports from nestbox studies: a review of inadequacies. Acta Ornithol 46:13–17
Wesołowski T, Cholewa M (2009) Climate variation and bird breeding season in a primeval temperate forest. Clim Res 38:199–208
Wesołowski T, Maziarz M (2012) Dark tree cavities—a challenge for hole nesting birds? J Avian Biol 43:454–460
Wesołowski T, Rowiński P (2004) Breeding behaviour of Nuthatch Sitta europaea in relation to natural hole attributes in a primeval forest. Bird Study 51:143–155
Wesołowski T, Rowiński P (2012) The breeding performance of Blue Tits Cyanistes caeruleus in relation to the attributes of natural holes in a primeval forest. Bird Study 59:437–448
Wesołowski T, Tomiałojć T (2005) Nest sites, nest depredation, and productivity of avian broods in a primeval temperate forest: do the generalisations hold? J Avian Biol 36:361–367
Wesołowski T, Czeszczewik D, Rowiński P, Walankiewicz W (2002) Nest soaking in natural holes—a serious cause of breeding failure? Ornis Fenn 79:132–138
Wesołowski T, Rowiński P, Mitrus C, Czeszczewik D (2006) Breeding bird community of a primeval temperate forest (Białowieża National Park, Poland) at the beginning of the 21st century. Acta Ornithol 41:55–70
Wesołowski T, Mitrus C, Czeszczewik D, Rowiński P (2010) Breeding bird dynamics in a primeval temperate forest over thirty five years: variation and stability in the changing world. Acta Ornithol 45:209–232
Winkel W (1970) Hinweise zur Art- und Altersbestimmung von Nestlingen höhlenbrütender Vogelarten anhand ihrer Körperentwicklung. Vogelwelt 91:52–59
Acknowledgments
We are very grateful to M. Czuchra and P. Rowiński for their participation in the fieldwork. The kind cooperation of the Białowieża National Park administration is acknowledged. We also thank Arie J. van Noordwijk, Emma Vatka and two anonymous reviewers for constructive comments on the manuscript. The presented data constitute part of an unpublished PhD thesis at the University of Wrocław (Maziarz 2012). Research was supported by internal grants from the faculty of biological sciences, University of Wrocław (TW, MC, MM) and EU Human Capital Programme (MM). The study complied with current Polish law and was permitted by the Ministry of the Environment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maziarz, M., Wesołowski, T., Hebda, G. et al. Breeding success of the Great Tit Parus major in relation to attributes of natural nest cavities in a primeval forest. J Ornithol 157, 343–354 (2016). https://doi.org/10.1007/s10336-015-1294-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1294-2