Abstract
We monitored population size from 1996 to 2003 and studied behavioural interactions (in 2001) between the native Black-headed Gull Chroicocephalus ridibundus and an expansive, opportunistic predator, the Caspian Gull Larus cachinnans, at water reservoirs in Poland. The expansive species caused a population decline in the native species and affected its choice of nest sites. The Black-headed Gulls perceived the risk of predation on the part of the larger Caspian Gulls. When both species occurred in close proximity, the native gull breeding pairs built nests where the vegetation was higher and its cover greater than at the sites chosen by pairs breeding far away from the expansive species. The native gulls in proximity to the expansive species spent more time guarding their nests. However, this was not compensatory, as egg losses were higher and breeding success much lower in pairs breeding near the Caspian Gulls than in those breeding far from the latter. Such a low breeding performance in the Black-headed Gulls was probably caused either by predation on the part of Caspian Gulls or by aggressive interactions among Black-headed Gulls. In fact, the rate of intraspecific aggression in native gulls was higher in pairs breeding in proximity to the expansive species than in those breeding far away from it. These intraspecific fights, caused by the presence of the expansive species were, at least partially, responsible for egg and chick losses. We did not find the presence of native gulls to have any effect on the behaviour and breeding performance of the expansive gull. These results indicate that the expansive predatory Caspian Gull negatively affects local population size and alters the behaviour of the native Black-headed Gull, and may, both directly and indirectly, affect its reproductive performance.
Zusammenfassung
Auswirkungen des Populationswachstums der expansiven Weißkopfmöwe Larus cachinnans auf Populationsgröße und Verhalten der Lachmöwe Chroicocephalus ridibundus
Wir haben die Populationsgröße der Lachmöwe Chroicocephalus ridibundus an polnischen Stauseen zwischen 1996 und 2003 erfasst und im Jahr 2001 zusätzlich Verhaltensinteraktionen mit einem opportunistischen Räuber, der Weißkopfmöwe Larus cachinnans, untersucht. Die expansive Weißkopfmöwe verursachte einen Populationsrückgang der heimischen Lachmöwe und beeinflusste ihre Nistplatzwahl. Die Lachmöwen nahmen das Prädationsrisiko durch die größeren Weißkopfmöwen wahr. Wenn beide Arten in unmittelbarer Nähe zueinander vorkamen, bauten die Lachmöwenpaare ihre Nester in höherer Vegetation, wo sie besser versteckt waren, verglichen mit Paaren, die weiter entfernt von Weißkopfmöwen brüteten. In der Nähe der expansiven Art verbrachten die heimischen Möwen mehr Zeit damit, ihre Nester zu bewachen. Dennoch hatten Lachmöwenpaare, die in der Nähe von Weißkopfmöwen brüteten, höhere Eiverluste und einen deutlich niedrigeren Bruterfolg als Paare, die weiter entfernt brüteten. Eine derart niedrige Fortpflanzungsleistung der Lachmöwen war wahrscheinlich entweder auf Prädation durch Weißkopfmöwen oder auf aggressive Interaktionen zwischen Lachmöwen zurückzuführen. In der Tat war die intraspezifische Aggression der Lachmöwen höher bei Paaren, die in der Nähe von Weißkopfmöwen brüteten, als bei Paaren, die weiter entfernt brüteten. Diese durch die Anwesenheit der Weißkopfmöwe verursachten intraspezifischen Kämpfe waren zumindest zum Teil für Ei- und Kükenverluste verantwortlich. Wir fanden keine Hinweise darauf, dass die Anwesenheit der heimischen Möwen das Verhalten und die Fortpflanzungsleistung der expansiven Möwe beeinflusste. Diese Ergebnisse deuten darauf hin, dass die expansive räuberische Weißkopfmöwe die lokale Populationsgröße der heimischen Lachmöwe negativ beeinflusst, ihr Verhalten verändert und sowohl direkt als auch indirekt ihre Fortpflanzungsleistung beeinflussen kann.
Similar content being viewed by others
Introduction
Predation is a process of major importance in biology, influencing the distribution, abundance, and behaviour of most of animals (Lima and Dill 1990; Lima 1998; Cervencl et al. 2011; Cresswell 2011). Predatory species typically exert top–down control on ecosystems through their direct predatory and competitive interactions with non-predatory species or smaller predators, as well as indirectly, through a trophic cascade (Frank et al. 2005; Ritchie and Johnson 2009). The effect of predation on ecosystem processes may be especially strong when predatory species are introduced from distinct geographical regions or arrive and expand their ranges, leading to population decline in native species and, therefore, to a substantial loss of biodiversity (Salo et al. 2007). The interactions between invasive or expansive species and native ones often constitute a completely new evolutionary situation for two, or more, species that have never coevolved and are confronted with each other over a short period of time (Mooney and Cleland 2001). This may cause the very rapid evolution of both invasive predators and native species (Huey et al. 2000; Phillips and Shine 2006; Suarez and Tsutsui 2008). Furthermore, when the expansive or invasive species are both competitors and opportunistic predators, they may have a particularly dramatic effect on ecosystems, since the wide range of native species is potential prey (Mooney and Cleland 2001; Finney et al. 2003; Rehage et al. 2005; Caut et al. 2008; Newson et al. 2010).
Many of the prey perceive the presence of predators and respond by modifying their behaviour or phenotype in order to reduce predation risk (Abrams 2000; Relyea 2003; Forstmeier and Weiss 2004; Morosinotto et al. 2010; Kryštofková et al. 2011). However, the lack of coevolutionary history between native and invasive predatory species raises the question as to whether or not the mechanism of competition and predation avoidance works in native species. When a competitor and predator appears in new areas, the native species may be unable to perceive a new risk or may perceive the risk but respond maladaptively (Mooney and Cleland 2001; Sih et al. 2010).
Among birds, many gull species spread to new areas at the end of the twentieth century (e.g. Burger and Lesser 1980; Wilds and Czaplak 1994; Vidal et al. 1998; Garthe et al. 1999; Thyen and Becker 2006; Lenda et al. 2010). In Europe, some species that originally occurred mostly on the coast expanded to inland areas where they had never occurred before (Hüppop and Hüppop 1999; Zielińska et al. 2007; Lenda et al. 2010). The main reasons for this wide range of expansion were the availability of trawler discards, anthropogenic refuse, and high breeding success in newly colonised areas (Fasola et al. 1993; Jonsson 1998; Skórka et al. 2005). Gulls, mostly large-bodied species, such as the Caspian Gull Larus cachinnans, are opportunistic predators inhabiting the same habitats, namely islets on bodies of water, as native waterbird species (Skórka et al. 2005; Lenda et al. 2010). Therefore, the presence of these expansive species may have important consequences for populations of native waterbirds. Large gulls may exclude native species from breeding sites and predate their eggs and chicks (Hario 1994; Skórka et al. 2005; Oro and Martinez-Abrain 2007). The risk of egg predation has led to the evolution of many forms of parental defence in animals, including gulls (Clutton-Brock 1991). Such defence can greatly increase hatching success (e.g. Bukacińska et al. 1996; Zink 2003); however, parental investments, including nest and chick defence, are also costly in terms of energy expenditure (e.g. Trivers 1972; Hario 1990; Hario et al. 1991; Wendeln and Becker 1999; Kokko and Jennions 2008).
In this study, we examined interactions between the Black-headed Gull Chroicocephalus ridibundus (BHG), a waterbird that is native to Central Europe, and the expansive large-bodied Caspian Gull (CG). The latter species has colonised inland reservoirs in Central Europe and excluded some native species, including BHG, from their breeding grounds (Skórka et al. 2005; Wójcik et al. 2005; Lenda et al. 2010). Like other large gulls, the CG is an opportunistic predator hunting the chicks of other waterbird species (Vidal et al. 1998; Guillemette and Brousseau 2001). First, we were interested in seeing whether the two species interact with each other and what impact the expansive CG has on the population size of native BHG breeding in the same reservoir. We were also interested in observing which of these two species is more successful in establishing a population when the availability of nesting space decreases. Second, we investigated whether the native species perceived the potential predator and changed its behaviour in such a way as to minimise egg and chick predation. We expected that, in places where these two species co-occur, the native BHGs would build nests in more concealed sites, namely with higher vegetation and a greater percentage of vegetation cover around the nests than occurs in sites where the invasive species is absent. For gulls, vegetation cover is positively related with predation avoidance and breeding success (Parsons and Chao 1983; Bosh and Sol 1998; Garcia-Borboroglu and Yorio 2004). We also expected that, where the expansive, predatory CGs were present, the native BHGs would guard their nests and chicks more intensively than in sites where they were absent, given that, in gulls, as in many other species, nest-guarding is positively correlated with breeding success (Bukacińska et al. 1996, 1998; Catry et al. 2010). Specifically, we predicted that, in the presence of CGs, the BHGs would guard their nests with eggs and chicks for a greater proportion of time and that interspecific aggressive behaviour would be displayed predominantly, as compared to sites without CGs.
Methods
Study area
The study was carried out on one of the largest CG colonies in Poland, with 177 pairs in 2001 (Skórka et al. 2005). It is located in Tarnów, in the south of the country, at a water reservoir of 56 ha (Fig. 1, Skórka et al. 2005). The CGs nested sympatrically with a large number of BHGs (up to 2,782 pairs in 1996) on 85 small islets of between 1 and 50 m2 and a larger islet of 1 ha (Fig. 1).
Location of the study area and design of the behavioural study of interactions between native Black-headed Gulls Chroicocephalus ridibundus and expansive Caspian Gulls Larus cachinnans. In 2001, 985 Black-headed Gull breeding pairs and 82 Caspian Gull pairs bred on the largest islet in the study colony. Black-headed Gulls occupied approximately two-thirds of the islet and Caspian Gulls occupied one-third of it, with a contact zone where the both species occurred in close proximity. Plots (5 × 20 m) were established in the contact zone and in the control areas for both species, where only conspecifics bred
Numerical response of the BHGs to the population growth of the CGs
We monitored the breeding population size of BHGs and CGs at the reservoir and two control reservoirs of similar size between 1996 and 2003. The control reservoirs, from which CGs were absent, were located 1 km south and 70 km west, respectively (see also Skórka et al. 2005).
Islets on inland reservoirs are a limited resource (Skórka et al. 2005; Lenda et al. 2010). We thus also observed the response of both species to the reduction of nest site availability. Furthermore, we took advantage of a ‘natural experiment’ which occurred in our study area. At the end of 2001, the flooding of the reservoir with additional water began, resulting in the reduction of available space by 80% in 2003; the total area of islets decreased from 12,080 to 2,400 m2 and 73 (86%) of the 85 islets disappeared, while the area of the largest islet decreased from 10,009 to 2,053 m2. We compared, in percentages, the extent of the decrease in population size of CGs and BHGs after the reservoir was flooded with additional water. We assumed that, in both species, the decrease in the number of breeding pairs should be proportional to the decrease in the availability of nesting sites.
Nest-site selection in BHGs and CGs
In order to study the effects of the CGs’ presence on the BHGs’ nest site selection, behaviour and breeding performance, in 2001 we established four sample plots, two for each species, on the largest of the reservoir’s islands (Fig. 1). This islet lay at the centre of the gull colony on this reservoir and 6,740 m2 of it was occupied by BHGs (989 pairs) and 3,260 m2 by CGs (82 pairs). There were two plots in the contact zone, in other words, the area where both species bred close to each other, one for the BHGs and the second for the CGs (Fig. 1). These plots were established in the part of the contact zone where the BHGs’ and CGS’ area met in a straight line, with no mixing of species. The remaining two plots were control plots, one for BHGs and one for the CGs. These were located 20 m from the plots in the area where both species occurred in close proximity. In the control plots, the birds were only involved in intraspecific interactions.
Each plot was 20 m long and 5 m wide and divided into four subplots (5 × 5 m). The area of all the plots and the distances between them were chosen in such a way as to retain a similarity in terms of nest density within species) and of vegetation structure. The dominant vegetation was patches of grasses, mostly Feather Reed Grass Calamgrostis epigeios, co-occurring with Stinging Nettle Urtica dioica. The boundaries of the plots were marked with wooden sticks. In each plot, all the nests of both species were marked, and nest histories (egg laying date, egg fate and hatching success) were determined on the basis of visits carried out either every day or every second day during incubation and hatching periods. At each nest, we measured vegetation height and vegetation cover in a 50-cm radius at the beginning of May. Vegetation height was measured at ten points within a 50-cm radius and the mean measurement from the points was used in further analyses. Vegetation cover was measured by the vertical projection of the vegetation and the bare ground within a 50-cm radius around the nest and transferred onto graph paper. Then, vegetation cover was calculated using planimetry. The same parameters were taken for a sample of random points within each plot. In the case of the CGs, we also measured vegetation at a few additional nests located near the plot in the contact zone and control plot, in order to receive a meaningful sample size.
Behaviour of species
For all the plots, we used a hide for observing the behaviour of randomly selected pairs to provide a basis for establishing the nest attendance pattern and calculating the occurrence rate of aggressive conflicts during the incubation and chick-rearing period. We endeavoured to maintain an equal amount of observation time among pairs. We therefore devoted six observation sessions to both the incubation and chick-rearing periods. Three of these sessions took place the morning, from 0600 to around 1000 hours and three in the afternoon, from 1200 to 1600 hours. Thus, each pair was observed during six sessions during the incubation period and six sessions during the chick-rearing period. One pair was observed for approximately 1 h during one session. Observation of the plot was always carried out by two observers. However, the total time devoted to the observations differed slightly between pairs (see below), since, under adverse weather conditions, the observations were necessarily aborted. Those of the selected nests that were close to each other were observed simultaneously by one observer, who monitored the selected nests assigned to him or her continuously during the session and noted the behaviour of birds.
On average, in the zone where two species co-occurred, we spent 409.4 ± 82.6 (mean ± SD) min on behavioural observations per pair of BHGs during incubation (n = 12 pairs) and 311.5 ± 62.0 min during the chick-rearing period (n = 8 pairs). For the control plot where only BHGs were breeding, we spent on average 405.4 ± 74.1 min on behavioural observations during incubation (n = 15 pairs) and 297.8 ± 71.4 during the chick-rearing period (n = 14 pairs).
In the case of the CGs breeding in the contact zone, we spent, on average, 398.5 ± 65.3 min on behavioural observations per pair during incubation (n = 6 pairs) and 266.7 ± 53.2 min during the chick-rearing period (n = 6 pairs). In the control plot where only CGs were breeding, we spent, on average, 390.0 ± 50.2 min on behavioural observations during incubation (n = 6 pairs) and 310.0 ± 70.1 during the chick-rearing period (n = 6 pairs).
Aggressive behaviour in gulls is complex (Groothuis 1989a, b). Therefore, we only took into consideration overt, highly aggressive behaviour, in other words, fights and aggressive attacks towards neighbours, since these were easily distinguishable in field conditions. A fight was defined as being when one bird rushed towards another, primarily mostly in flight, and tried to peck it or jump onto its shoulders. Physical contact was always a factor in the fights. Aggressive attacks were very similar to fights, but here, the bird being attacked quickly ran away and there was thus no physical contact. We noted the duration (1) of both parents’ presence at the nest, (2) of one bird’s presence and (3) for which the nest was unattended.
Every second day, we surveyed all the nests within the study plots, counted all the eggs and marked them with an individual code. We noted every case where eggs disappeared, were crushed or rolled out of the nest. Hatching success was estimated in two ways: firstly, as the proportion of eggs that hatched from among those that survived to hatching time, and secondly, as the mean number of chicks hatched per pair. Egg losses were estimated as the proportion of eggs that disappeared, rolled away or were crushed to the total number that were laid.
In order not to disturb the behaviour of the birds, and to minimise the possible effects arising from the presence of the observers, the nests were not fenced (e.g. Oro et al. 1996; Jehl 2001; Ležalová-Piálková 2011). Therefore, to determine fledging success we applied the following two procedures:
-
1.
First, from the hide, we counted all the BHG chicks and CG chicks present at their nests when they were at an average age of 2 and 3 weeks, respectively.
-
2.
Counting fledglings from the hide may underestimate fledging success, especially when young birds hide in grass tufts. Therefore, after counting from the hide, all the chicks at nests within the plots were marked on their bills with Tipp-ex, a non-toxic white marker that disappears after a few days. Two hours later, we counted all the chicks present at the nests within the sample plots from the hide to determine the number of both marked and unmarked chicks (Table 1). The modified Lincoln–Petersen method (Seber 1982; Krebs 1989; Martinez-Abrain et al. 2003) was then used to determine the number of chicks in the plots:
$$ N = \frac{(M + 1)(C + 1)}{R + 1} - 1, $$where N is the estimated total number of fledglings, M is the number of fledglings at nest and marked with Tipp-ex, and C is the total number of chicks observed at the nests after marking, including R, the number of chicks marked (Table 1).
Of course, the method assumes a closed population, while the plots were not fenced. However, the chicks stayed close to their nests and we could therefore assume that they constitutes a closed population, even though this was not formally the case (see Kendall 1999 for discussion on this issue). The plots were located at a considerable distance from the water and, thus, the chicks would not have escaped into the reservoir. The counting of chicks took place very rapidly, taking no more than approximately 10 min, meaning that the likelihood of their being counted twice was slim. Besides, the potential bias should be the same in both the experimental and control plots and what was of interest to us were the relative differences rather than the precise real estimates.
Fledging success was estimated as the number of chicks with an average age of 2 weeks (BHGs) or 3 weeks (CGs) divided by the number of chicks hatched. The total breeding success was estimated as the number of chicks with an average age of 2 weeks (BHGs) or 3 weeks (CGs) divided by the number of eggs laid. Calculations of the fledging and total breeding successes were based on the numbers of fledglings counted from the hide and the numbers of fledglings estimated by the capture-mark-resight method (Table 1).
Statistics
We used a bootstrapped correlation analysis to test the statistical significance of the population size changes in the two species. A generalised linear model (GLM) with an identity link function was used to test the differences in population trends in the BHGs inhabiting the invaded and the two control reservoirs. The interaction between the reservoirs’ identities (invaded, control 1 and control 2) and year of study was of primary interest, because this term tested the statistical significance of the difference in BHG population trends in the invaded and the control reservoirs. The chi-square test was used to test the effects of the reduction of nesting space on the number of breeding pairs in the two species. We tested whether the expected frequencies correspond to the observed ones. To compare the proportion of eggs lost and the proportion of chicks that hatched in the control plot and the plot in the contact zone in both species, the generalised linear mixed model (GLMM) with logit link function and binomial error variance was used. This model was also used to compare the proportion of BHG eggs that were outside their natal nests, crushed and disappeared in the control plot and the plot in the contact zone. Nest identity was assigned as a random effect in these models. The GLM with logit link function and binomial error variance was used to compare fledging success and total breeding success in the birds breeding in the control plot and in the contact zone. A bootstrapped t test was used to compare the mean date of clutch initiation, mean nest density, mean clutch volume, mean number of hatched chicks per nest, proportion of time when two parents attended the nest and when the nest was unattended in pairs breeding in the control plot and the plot in the contact zone. This test was also used to compare the mean proportion of time when two parents attended the nest and when the nest was unattended between incubation and chick-rearing period in both species. In the case of the BHGs, the comparison of the proportion of time spent at the nests between incubation and the chick-rearing period was carried out using t tests for independent samples, rather than a t test for matched pairs, because many of the pairs observed during incubation lost their broods and the sample size was therefore lower during the chick-rearing period. The bootstrapped t test was also used to compare the rate of aggressive encounters in pairs breeding in plots in the contact zone and in the control area for both species. A bootstrapped one-way analysis of variance was applied to compare vegetation height and vegetation cover at nest and random points between the plot in the contact zone and the control plot for both species. We used the bootstrapped correlation, t test and one-way analysis of variance because these tests are preferred over ordinary equivalents when sample sizes are small or unequal or when the data distribution is not known (Good 2005; Edgington and Onghena 2007; Manly 2007), as occurred for many of the cases in our data set.
The GLM and GLMM were performed using SPSS v.19 (IBM, Somers, NY, USA) software. All correlation analyses, t tests, and ANOVA were performed in Rundom Pro 3.12 (Jadwiszczak 2009).
Results
Numerical response of BHGs to population growth of CGs
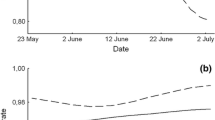
We found that the BHG population size decreased, while the population size of CG increased (r = −0.912, P = 0.003, n = 6 years) until 2001, when the flooding of the reservoir with additional water began (Fig. 2). Simultaneously, the BHG population sizes for the control reservoirs increased (interaction between year and identity of the reservoir in GLM F 2,23 = 96.421, P < 0.001; Fig. 2). We found that both species decreased in population size, but to a different degree, after reduction of breeding islet availability (Fig. 3). After reduction of the islets’ area, the relative decrease in the number of pairs was greater in BHG than in CGs (χ 21 = 109.259, P < 0.001; Fig. 3).
Exclusion of the Black-headed Gulls by the Caspian Gulls. a Local population growth of the Caspian Gull, b local population decrease in Black-headed Gulls (circles). In the control reservoirs (triangles and squares), where Caspian Gulls were absent, the population sizes of Black-headed Gulls were increasing. The arrow indicates year when flooding of the study area started
Natural experiment showing that expansive Caspian Gulls (CGs) deal better with a situation of the limited nesting space than native Black-headed Gulls (BHGs). a In 2001, as many as 1,178 pairs of BHGs and 177 pairs of CGs breed in the study reservoir. In 2001, the filling of the reservoir with water surplus began, reducing the nesting space by 80% in 2003. b Reduction of the nesting space should result in a proportional reduction of population sizes of both species, as indicated by the grey bars. The white bars indicate the real numbers of breeding pairs
We also observed that the number of BHG nests located on the shore of the reservoir increased as the CG population size grew (r = 0.966, P = 0.002, n = 6 years) and reached a maximum (n = 22 nests) in 2001. All these nests were predated by corvids and foxes. Moreover, in the years with the highest number of CGs, we also noted seven cases of BHG nests built in old Magpie (Pica pica) nests in the trees along the shore.
Nest-site selection
BHG nest density did not differ in the plot near the CGs and the control plot (Table 2). The BHGs built nests in places with greater vegetation cover around the nests (one-way ANOVA F 3,168 = 130.123, P < 0.001, n = 172) in the presence of CGs than they did in the control plot (post hoc test, P < 0.001; Fig. 4). Both BHGs and CGs (one-way ANOVA F 3,108 = 10.228, P < 0.001, n = 112) built nests in greater vegetation cover than was found for random points (both post hoc tests significant at the P < 0.001; Fig. 4).
Choice of vegetation cover around nests by a native Black-Headed Gulls (BHGs) and b expansive Caspian Gulls (CGs) in the contact zone where both species occurred in close proximity and in the control plots, where only conspecifics were present. Means are shown with 95% confidence intervals. Samples sizes are given in parentheses. ***P < 0.001
In the proximity of the CGs, the BHGs built their nests in higher vegetation than noted at the random points (one-way ANOVA F 3,168 = 5.831, P < 0.001, n = 172 with post hoc test for this specific comparison significant at P = 0.009) and this vegetation was higher around the nest of the BHGs in the proximity of CGs than around nests in the control plot (post hoc test P = 0.010; Fig. 5).
Choice of vegetation height around nests by a native Black-headed Gulls (BHGs) and b expansive Caspian Gulls (CGs) in the contact zone where both species occurred in close proximity and in control plots. *P < 0.05, **P < 0.01. For further explanations, see Fig. 4
We found no effect of the presence of BHGs on nest-site selection in CGs (Figs. 4 and 5). Vegetation cover did not differ between random points in the plot in the contact zone and the control area for either BHGs or CGs (Fig. 4). Similarly, as far as vegetation height was concerned, the random points did not differ between control plots and plots in the contact zone for either BHGs or CGs (Fig. 5).
Nest attendance pattern
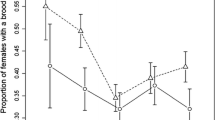
During the incubation period, the proportion of time when both BHG parents were present at nests in the contact zone was greater than in the control plot (t = 2.311, P = 0.033, n = 12 pairs in contact zone and 15 pairs in the control plot; Fig. 6). During the chick-rearing period, adult BHGs shared parental duties in the contact zone; we noted a lower proportion of time when the nest was unattended compared to the control plot (t = −4.667, P = 0.003, n = 8 pairs in plot in the contact zone and 14 pairs in the control plot; Fig. 6). Simultaneously, the proportion of time when both parents were present was shorter compared to the incubation period (t = 2.920, P = 0.013, n = 12 pairs examined during incubation and eight pairs during the chick-rearing period; Fig. 6). In contrast, at the control plot, the proportion of time when two BHG parents were present at the nest was higher during the chick-rearing period than during incubation (t = −2.215, P = 0.046, n = 15 pairs examined during incubation and 14 pairs during the chick-rearing period; Fig. 6), but the proportion of time when the nest was unattended was higher during the chick-rearing period than during incubation (t = −5.352, P < 0.001, sample size as in the previous test; Fig. 6).
Nest guarding in a Black-headed Gulls (BHGs) and b Caspian Gulls (CGs). The white bars indicate pairs breeding in the contact zone, in proximity of other species, the grey bars indicate pairs breeding in the control plots, solely among conspecifics. In the contact zone, 12 and 8 pairs of BHGs were examined during incubation and the chick-rearing period, respectively, and 15 and 14 pairs for both breeding stages were examined in the control plot. Six pairs of CGs were examined during the two breeding stages in both the contact zone and control plot. For further explanations, see Figs. 4, and 5
We found no significant effect of the proximity of BHGs on the CG nest attendance pattern (Fig. 6). For CGs, there were also statistically significant differences in nest guarding between the incubation and chick-rearing periods. Contrary to BHGs, the proportion of time when the nest was unattended was higher during the chick-rearing period than during incubation for both the plot in the contact zone (t = −5.298, P = 0.026, n = 6 pairs examined in both periods; Fig. 6) and the control plot (t = −3.609, P = 0.029, n = 6 pairs examined in both periods; Fig. 6).
Breeding performance
The date of clutch initiation and clutch volume was similar for BHGs breeding in the proximity of CGs and those breeding in the control plot (Table 2). However, the proportion of abandoned nests was higher in the plot in the contact zone than in the control plot, while the hatching, fledging and total breeding successes were considerably lower. When we carried out a detailed scrutiny of the cases of BHG nest failure, we found significantly more eggs which had rolled out of the nests and crushed eggs in the plot in the contact zone than in the control plot (Table 3). The proportion of eggs that disappeared was low and similar in both plots (Table 3).
We found no significant effect of the proximity of BHGs on the CG’s breeding performance (Table 4).
Rate of aggressive encounters
Although the BHGs displayed compensatory behaviour in the proximity of CGs, such as breeding in denser and taller vegetation, and evincing better nest guarding behaviour, the breeding performance, as shown above, still remained low. We therefore compared intra- and interspecific aggressive behaviour of the species. Surprisingly, we found that the BHGs in the proximity of CGs showed a rate of intraspecific aggressive encounters that was almost six times higher than in the control plot during incubation (t = 13.007, P < 0.001, n = 12 pairs examined in the plot in the contact zone and 15 pairs in the control plot) and during chick-rearing periods (t = 12.124, P < 0.001, n = 8 pairs examined in the plot near CGs and 14 pairs in the control plot; Fig. 7). However, the aggression was interspecific and directed towards CGs. Some 60% of intraspecific aggression events (n = 1,561 intraspecific aggression events in BHGs in the plot in the contact zone) occurred immediately after BHG aggression towards CGs. We found a positive correlation between intra- and interspecific aggressive encounter rates within BHG territories both during incubation (r = 0.711, P = 0.010, n = 12 territories) and during the chick-rearing period (r = 0.730, P = 0.035, n = 8 territories). During field observation, we noted that the appearance of CGs in the close vicinity of BHG nests, even for short periods of time, such as, for example, when CGs landed among BHG nests and walked to their own, caused a great deal of panic among BHGs. They immediately chased the CGs away, but violent conflicts among neighbouring BHGs arose simultaneously, apparently as a result of the violation of territorial boundaries. In effect, every appearance of CGs gave rise to a ‘wave’ of intraspecific aggression among BHGs. This was only observed in the plot in the contact zone.
In general, during the BHGs’ intraspecific conflicts with their neighbours, many eggs rolled out of the nests or were crushed; during such situations, we directly observed n = 12 eggs rolled away and n = 4 crushed. During the chick-rearing period, aggression towards chicks was also visible; we directly observed n = 8 cases that ended with a chick’s death. However, it was never observed in the control plot.
We found no significant effect of the presence of BHGs on the aggressive behaviour of CGs (Fig. 7).
Discussion
As we have demonstrated, the expansive CGs negatively affected the local population size of native BHGs. The local CG population grew rapidly, even though the BHGs were far more abundant. Three complementary mechanisms may explain this result. Firstly, the CG is a large-bodied species and may be a stronger competitor for breeding sites than BHGs. Secondly, the CGs started laying eggs about 2 weeks earlier than the BHGs and thus excluded them from the breeding islets. Third, the native BHGs perceived CGs as a potential predator and could be reluctant to breed in their proximity.
Body size is one of the major indicators of competitive ability in animals (Alatalo and Moreno 1987; Lindstrom 1988; Jonart et al. 2007). Some smaller species, if abundant enough, are able to resist new colonisers and effectively compete with larger species, as was found with the Royal Tern Sterna maxima and Cayenne Tern S. eurygnatha when competing with larger Kelp gulls Larus dominicanus (Quintana and Yorio 1998). This, however, was not the case in our study system.
CGs are large birds and they may thus also outcompete other native waterbirds besides BHGs from islets. The islets are usually in shortage at inland reservoirs in our study region and are therefore one of the most limited resources for waterbirds (Skórka et al. 2005; Lenda et al. 2010).
The CGs started laying eggs 2 weeks before the BHGs. In fact, the CGs hold the breeding territories from the beginning of February, thus making them inaccessible to smaller and later-breeding species (Skórka et al. 2005). The BHGs, facing a shortage of nest sites, started to locate their nests on the shore and in old magpie nests, which inevitably resulted in egg losses (see also Burger 1979; O’Connell and Beck 2003). Not once did we observe BHGs breeding in the nests of other species at the control reservoirs, and neither have we seen this in other monospecific BHG colonies in Poland. This indicates that the BHGs were attached to the colony under study and sought out whatever spot they could find in order to breed there. Breeding site philopatry is widespread in gulls (Spear et al. 1998) and it may explain why the birds exhibited this odd behaviour. However, the decrease in the population size of BHGs in the invaded reservoir corresponded well with the simultaneous increase in the colony size of BHGs at the control reservoir located 1 km apart. This suggests that some birds could have left the natal colony and settled in the new reservoir. Such shifts in both small-scale nest-site choice and possible changes in colony location are very interesting, because they show that the expansive CGs may directly or indirectly increase the variation of breeding success in BHGs within the invaded local population and/or generate a system of BHG colonies similar to sink–source metapopulation (Pulliam 1988).
The difference in population trends between CGs and BHGs could also be attributable to a fear of the CGs’ presence. Large gulls are major predators of the eggs and chicks of other waterbirds and affect their breeding success and reproductive strategies (Kruuk 1964; Becker 1984; Hario 1994; Yorio and Quintana 1997). Smaller gull species usually display a high degree of coloniality and breed at high densities in large colonies which are prerequisites for successful colony defence against predators (Kruuk 1964; Tinbergen 1967; Fuchs 1977; Becker 1995). Because most of the islets in our study reservoir were small, they could only be inhabited by a few pairs of BHGs. Such small groups were probably less successful in defending the nests (see Becker 1984) on the islet against the CGs that overtook neighbouring islets and, therefore, it is possible that the BHGs moved to other areas. Moreover, it seems that CGs display a lower degree of coloniality than BHGs, with solitary pairs frequently found in newly colonised areas (Lenda et al. 2010).
Obviously, in the face of an increasing population of the expansive predator, the native species may possess anti-predator adaptations that include morphological and behavioural changes, reducing the probability of mortality and/or eggs and chick losses (Kiesecker and Blaustein 1997; Freeman and Byers 2006). In our study, the native BHGs were able to recognise the CGs as a potential threat to their broods and responded to the presence of the predatory species by changes in nest-site choice and prolonged nest guarding. These results are in line with the theory and data for other animals, which show that behavioural response to larger, potential predators often results in changes of microhabitat choice (Abrams 2000; Eggers et al. 2006; Fontaine and Martin 2006). In the presence of large gulls, smaller species build nests in sites with greater vegetation cover (Burger and Shisler 1978; Burger 1979) and demonstrate increased aggression (Cavanagh and Griffin 1993; Whittam and Leonard 2000). The important finding of our study, though, is that this response was non-compensatory. The hatching, fledging and total breeding success of the BHGs breeding near CGs were lower than in those breeding only among conspecifics. This result is even more unexpected as it is believed that taller vegetation and the higher cover reduce visual contact between neighbours and lessen antagonistic interactions between individuals (Burger 1977; Bukacińska and Bukaciński 1993; Sin-Yeon and Monaghan 2005). Our results are very similar to the data obtained by Becker (1984) in a colony of Common Terns Sterna hirundo under predatory pressure from Herring Gulls Larus argentatus. Up-flights of the entire colony of Common Terns occurred frequently and spontaneously during incubation, but were almost exclusively a response to the Herring Gulls attempting to predate their chicks. The lower the Herring Gulls flew over the colony, the more frequently the Common Terns flew up or attacked and the greater the number of individuals involved in these responses. However, despite the defence behaviour on the part of the terns, the Herring Gulls often succeeded in robbing them of their chicks and the breeding success of the Common Tern was poor (Becker 1984).
We could not exclude, though, the possibility that the lower breeding performance of the BHGs near the CGs was, to some degree, a result of a maladaptive response to the presence of the expansive predatory gull. We have shown that, by their panicked response to the proximity of the larger, invasive CGs, the native BHGs damaged their own broods. When nest density is high and territories very small, the vegetation cover and its height might not be enough to reduce aggression between neighbours. Many pairs of BHGs violated the boundaries of their neighbours, when trying to pursue CGs. This situation, in turn, leads to the increment of intra-species aggression and the increased mobility of BHG chicks, which are frequently attacked by neighbouring adults. In gulls, adults aggression towards trespassing chicks may be a major cause of chick mortality, as has been demonstrated in Glaucous-winged Gull Larus glaucescens chicks (Hunt and Hunt 1976).
In this study, we did not manage to document direct CG predation on the BHG broods in the contact zone. Our previous studies carried out in the same colony showed that the expansive CGs foraged mostly on fish, but chicks of BHGs were found at some nests (Skórka et al. 2005; Skórka and Wójcik 2008), and we also observed CGs hunting BHG chicks in other parts of the reservoir (authors’ unpublished data). Predation must, therefore, have been involved in such responses of panic to the presence of CGs. Detecting the occurrence of predation by larger larids on the chicks of smaller ones is difficult, because usually no more than a few individuals in the colony are true predators (Parsons 1971; Southern and Southern 1984; Hario 1994). Guillemette and Brousseau (2001) showed that, in the colony of Common Terns, large gulls predated 60% of chicks and just one individual was responsible for 85% of predation events. Most predation events occur on broods located near a predatory neighbour and, after the predators have been removed, new predators may appear (Guillemette and Brousseau 2001), some of which can come from longer distances and are even more difficult to detect (Hario 1994). The attacks carried out by large gulls are of short duration and difficult to establish. Moreover, the chicks of small gulls are soft-bodied prey and swallowed whole, so few remains can be found later. In our study colony, many of the CGs’ nests were located close to water. Most of the regurgitates thus simply drowned and this could also make for an underestimation of the predation impact of CGs on BHG chicks (Skórka et al. 2005).
We could not attribute the differences in breeding success in the native BHGs to the quality or experience of individual BHGs breeding in plots close to or distant from CGs. The clutch initiation date, volume of eggs and clutch sizes are often linked to the quality and body conditions of the birds (Nol et al. 1997; Wendeln and Becker 1999; Arnold et al. 2006; Wiebe and Bortolotti 2009; Hipfner et al. 2010). There is evidence that, in several gull species, high quality individuals with high breeding success start broods earlier and lay larger eggs (Davis 1975; Sydeman et al. 1991; Brouwer et al. 1995; Bukacińska et al. 1996; Kilpi et al. 1996). In our study, the BHGs breeding in the two plots had a similar clutch initiation date and similar numbers of eggs and clutch volumes, suggesting that the quality and experience of the birds breeding on these plots was similar. Also, the island in question was located at the centre of the gull colony, and the observed differences could not thus be attributed to differences between birds breeding at the colony’s centre and at its edge (Patterson 1965; Coulson 1968; Becker 1995; Cote 2000).
We believe that our method for the determination of fledging success in the BHGs was reliable. It differed from the more usually applied method of ringing chicks with a unique code and was chosen in order to minimise the negative effects of observers’ activity on chick behaviour. In this method, estimations of population size are based on proportions that are especially biased when the sample size is low. However, in both the control plot and the contact zone, the number of chicks resighted was large. Counting the chicks took less than 10 min, and thus the probability that some chicks were counted twice was, in all likelihood, low. Moreover, there is no indication that the bias of breeding success estimation in the control plots is larger than in the plot near the CGs (see Krebs 1989; Brower et al. 1998; Kendall 1999).
When species colonise new areas, they may experience an array of novel selective pressures and simultaneously act as novel selective agents on native taxa in the invaded ecosystem. However, we have shown that the native BHGs had no visible effect on the behaviour and reproduction of the invasive CGs. This contradicts the general view that native species affect the fitness components, that is, the reproductive success and parental effort of expansive or invasive species (Phillips and Shine 2006; Suarez and Tsutsui 2008). It is possible that expansive CGs possess traits that predispose them to exploit new areas and compete with and/or predate on native species successfully, since, in their original geographical range, namely the Black and Caspian Sea Basins, the CG co-occurs in breeding grounds with other, smaller gull species. On the other hand, for a long time, the native BHG was the only breeding gull species in the inland areas of Central Europe. The presence and nesting of large gulls is quite a new phenomenon in these areas (Filchagov 1996; Hüppop and Hüppop 1999; Skórka et al. 2005). Therefore, the expansion of CGs may constitute a new important factor negatively affecting local population size and breeding success of both the native BHGs and, probably, other native waterbirds.
References
Abrams PA (2000) The evolution of predator-prey interactions: theory and evidence. Annu Rev Ecol Syst 31:79–105
Alatalo RV, Moreno J (1987) Body size, interspecific interactions, and use of foraging sites in tits (Paridae). Ecology 68:1773–1777
Arnold JM, Hatch JJ, Nisbet IC (2006) Effect of egg size, parental quality and hatch-date on growth survival of common tern Sterna hirundo. Ibis 148:98–105
Becker PH (1984) Wie richtet eine FlußseeschwalbenKolonie (Sterna hirundo) ihr Abwehrverhalten auf den Feinddruck durch Silbermöwen (Larus argentatus) ein. Z Tierpsychol 66:265–288
Becker PH (1995) Effects of coloniality on gull predation on common tern (Sterna hirundo) chicks. Colon Waterbirds 18:11–22
Bosh M, Sol D (1998) Habitat selection and breeding success in yellow-legged gulls Larus cachinnans. Ibis 140:415–421
Brouwer A, Spaans AL, de Witt AAN (1995) Survival of herring gull Larus argentatus chicks: an experimental analysis of the need for early breeding. Ibis 137:272–278
Brower JE, Zar JH, von Ende CN (1998) Field and laboratory methods for general ecology, 4th edn. McGraw-Hill, Boston
Bukacińska M, Bukaciński D (1993) The effect of habitat structure and density of nests on territory size and territorial behaviour in the black-headed gull (Larus ridibundus L.). Ethology 94:306–316
Bukacińska M, Bukaciński D, Spaans AL (1996) Attendance and diet in relation to breeding success in herring gulls (Larus argentatus). Auk 113:300–309
Bukaciński D, Bukacińska M, Spaans AL (1998) Experimental evidence for the relationship between food supply, parental effort and chick survival in the lesser black-backed gull Larus fuscus. Ibis 140:422–430
Burger J (1977) Role of visibility in nesting behaviour of Larus gulls. J Comp Physiol Psychol 91:1347–1358
Burger J (1979) Competition and predation: herring gulls versus laughing gulls. Condor 81:269–277
Burger J, Lesser F (1980) Nest site selection in an expanding population of herring gulls. J Field Ornithol 51:270–280
Burger J, Shisler J (1978) Nest-site selection and competitive interaction of herring and laughing gulls in New Jersey. Auk 95:252–266
Catry P, Phillips RA, Forster IP, Matias R, Lecoq M, Granadeiro P, Strange IJ (2010) Brood-guarding duration in black-browed albatrosses Thalassarche melanophris: temporal, geographical and individual variation. J Avian Biol 41:460–469
Caut S, Angulo E, Courchamp F (2008) Dietary shift on an invasive predator: rats, seabirds and sea turtles. J Appl Ecol 4:428–437
Cavanagh PM, Griffin CR (1993) Responses of nesting common terns and laughing gulls to flyovers by large gulls. Wilson Bull 105:333–338
Cervencl A, Esser W, Maier M, Oberdiek N, Thyen S, Wellbrock A, Exo K-M (2011) Can difference in incubation patterns of common redshanks Tringa totanus be explained by variations in predation risk? J Ornithol 152:1033–1043
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Cote SD (2000) Aggressiveness in king penguins in relation to reproductive status and territory location. Anim Behav 59:813–821
Coulson JC (1968) Differences in the quality of birds nesting in the centre and on the edges of a colony. Nature 217:478–479
Cresswell W (2011) Predation in bird populations. J Ornithol 152(Suppl 1):S251–S263
Davis JWF (1975) Age, egg-size and breeding success in the herring gull Larus argentatus. Ibis 117:460–473
Edgington ES, Onghena P (2007) Randomization tests, 4th edn. Chapman & Hall/CRC, London
Eggers S, Griesser M, Nystrand M, Ekman J (2006) Predation risk induces changes in nest site selection and clutch size in the Siberian jay. Proc R Soc Lond B 273:701–706
Fasola M, Goutner V, Walmsley J (1993) Comparative breeding biology of the gulls and terns in the four main deltas of the northern Mediterranean. In: Aguilar JS, Monbailliu X, Paterson AM (eds) Status and conservation of seabirds. Ecogeography and Mediterranean Action Plan. SEO, GOB, Medmaravis, pp 111–123
Filchagov AV (1996) Colonization of the central part of the East-European plain by Larus argentatus-cachinnans. Ibis 138:148–150
Finney SK, Harris MP, Keller LF, Elston DA, Monaghan P, Wanless S (2003) Reducing the density of breeding gulls influence the pattern of recruitment of immature Atlantic puffins Fratercula arctica to a breeding colony. J Appl Ecol 40:545–552
Fontaine JJ, Martin TE (2006) Parent birds assess nest predation risk and adjust their reproductive strategies. Ecol Lett 9:428–434
Forstmeier W, Weiss I (2004) Adaptive plasticity in nest-site selection in response to changing predation risk. Oikos 104:487–499
Frank KT, Petrie B, Choi JS, Leggett WC (2005) Trophic cascades in a formerly cod-dominated ecosystem. Science 308:1621–1623
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833
Fuchs E (1977) Predation and anti-predator behaviour in a mixed colony of terns Sterna sp. and black-headed gulls Larus ridibundus with special reference to the Sandwich tern Sterna sandvicensis. Ornis Scand 8:17–32
Garcia-Borboroglu P, Yorio P (2004) Effects of microhabitat preferences on kelp gull Larus dominicanus breeding performance. J Avian Biol 35:162–169
Garthe S, Freyer T, Hüppop O, Wölke D (1999) Breeding lesser black-backed gulls Larus graellsii and herring gulls Larus argentatus: coexistence and competition? Ardea 87:227–236
Good P (2005) Permutation, parametric, and bootstrap test of hypotheses, 3rd edn. Springer, Berlin
Groothuis T (1989a) On the ontogeny of display behaviour in the black-headed gull. I. The gradual emergence of adult forms. Behaviour 109:76–123
Groothuis T (1989b) On the ontogeny of display behaviour in the black-headed gull: II. Causal links between the development of aggression, fear and display behaviour: emancipation reconsidered. Behaviour 110:161–204
Guillemette M, Brousseau P (2001) Does culling predatory gulls enhance the productivity of breeding common terns? J Appl Ecol 38:1–8
Hario M (1990) Breeding failure and feeding conditions of lesser black-backed gulls Larus f. fuscus in the Gulf of Finland. Ornis Fenn 67:113–129
Hario M (1994) Reproductive performance of the nominate lesser black-backed gull under the pressure of Herring Gull predation. Ornis Fenn 71:1–10
Hario M, Kilpi M, Selin K (1991) Parental investment by the sexes in the herring gull: the use of energy reserves during early breeding. Ornis Scand 22:308–312
Hipfner MJ, McFarlane-Tranquilla LA, Addison B (2010) Experimental evidence that both timing and parental quality affect breeding success in a zooplanktivorous seabird. Auk 127:195–203
Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309
Hunt GL Jr, Hunt MW (1976) Gull chick survival: the significance of growth rates, timing of breeding and territory size. Ecology 57:62–75
Hüppop O, Hüppop K (1999) The food of breeding herring gulls Larus argentatus at the lower river Elbe: does fish availability limit inland colonisation? Atlantic Seabirds 1:27–42
Jadwiszczak P (2009) Rundom Pro 3.14. Software for classical and computer-intensive statistics available free from the New Rundom Site http://pjadw.tripod.com
Jehl JR Jr (2001) Enhanced success of California gulls nesting in enclosures. Waterbirds 24:133–136
Jonart LM, Hill GE, Badyaev AV (2007) Fighting ability and motivation: determinants of dominance and contest strategies in females of passerine bird. Anim Behav 74:1675–1681
Jonsson L (1998) Yellow-legged gulls and yellow-legged herring gulls in the Baltic. Alula 3:74–100
Kendall WL (1999) Robustness of closed capture-recapture methods to violations of the closure assumption. Ecology 80:2517–2525
Kiesecker DG, Blaustein AR (1997) Population differences in responses of red-legged frogs (Rana aurora) to introduced bullfrogs (Rana catesbeiana). Ecology 78:1752–1760
Kilpi M, Hillstrom L, Lindstrom K (1996) Egg-size variation and reproductive success in the herring gull Larus argentatus: adaptive or constrained size of the last egg? Ibis 138:212–217
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Krebs CJ (1989) Ecological methodology. Harper Collins, New York
Kruuk H (1964) Predator and anti-predator behaviour of the black headed gull (Larus ridibundus). Behaviour 11:S1–S29
Kryštofková M, Haas M, Exnerová A (2011) Nest defense in blackbirds Turdus merula: effect of predator distance and parental sex. Acta Ornithol 46:55–63
Lenda M, Zagalska-Neubauer M, Neubauer G, Skórka P (2010) Do invasive species undergo metapopulation dynamics? A case study of the invasive Caspian gull, Larus cachinnans, in Poland. J Biogeogr 37:1824–1834
Ležalová-Piálková R (2011) Molecular evidence for extra-pair paternity and intraspecific brood parasitism in the black-headed gull. J Ornithol 152:291–295
Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioural, reproductive and ecological perspectives. Adv Stud Behav 27:215–290
Lima SL, Dill LM (1990) Behavioural decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lindstrom K (1988) Male-male competition for nest sites in the sand goby, Pomatoschistus minutus. Oikos 53:67–73
Manly BFJ (2007) Randomization, bootstrap and Monte Carlo methods in biology, 3rd edn. Chapman & Hall/CRC, London
Martinez-Abrain A, Oro D, Izquierdo J, Ferris V, Belenguer R (2003) A comparison of two methods to estimate breeding productivity in a colonial ground-nesting gull Larus cachinnans. Marine Ornithol 31:71–74
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci USA 98:5446–5451
Morosinotto C, Thomson RL, Korpimaki E (2010) Habitat selection as an antipredator behaviour in a multipredator landscape: all enemies are not equal. J Anim Ecol 79:327–333
Newson SE, Leech DI, Hewson CM, Crick HQP, Grice PV (2010) Potential impact of grey squirrels Sciurus carolinensis on woodland bird populations in England. J Ornithol 151:211–218
Nol E, Blanken MS, Flynn L (1997) Sources of variation in clutch size, egg size and clutch completion dates on semipalmated plovers in Churchill, Manitoba. Condor 99:389–396
O’Connell TJ, Beck RA (2003) Gull predation limits nesting success of terns and skimmers on the Virginia barrier islands. J Field Ornithol 74:66–73
Oro D, Martinez-Abrain A (2007) Deconstructing myths on large gulls and their impact on threatened sympatric waterbirds. Anim Conserv 10:117–126
Oro D, Jover L, Ruiz X (1996) The influence of trawling activity on the breeding ecology of a threatened seabird, Audouin’s gull Larus audouinii. Mar Ecol Prog Ser 139:19–29
Parsons J (1971) Cannibalism in Herring Gulls. Br Birds 64:528–537
Parsons KC, Chao J (1983) Nest cover and chick survival in herring gulls. Colon Waterbirds 6:154–159
Patterson IJ (1965) Timing and spacing of broods in the black-headed gulls Larus ridibundus. Ibis 107:432–459
Phillips BL, Shine R (2006) An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc R Soc Lond B 273:1545–1550
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661
Quintana F, Yorio P (1998) Competition for nest sites between kelp gulls (Lars dominicanus) and terns (Sterna maxima and S. eurygnatha) in Patagonia. Auk 115:1068–1071
Rehage JS, Barnett BK, Sih A (2005) Behavioural responses to a novel predator and competitor of invasive mosquitofish and their non-invasive relatives (Gambusia sp.). Behav Ecol Sociobiol 57:256–266
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc Lond B 274:1237–1243
Seber GA (1982) The estimation of animal abundance and related parameters. Griffin, London
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser EL, Rehage JS, Vonesh JR (2010) Predatorprey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Sin-Yeon K, Monaghan P (2005) Interacting effects of nest shelter and breeder quality on behaviour and breeding performance of herring gulls. Anim Behav 69:301–306
Skórka P, Wójcik JD (2008) Habitat utilisation, feeding tactics and age related feeding efficiency in the Caspian gull Larus cachinnans. J Ornithol 149:31–39
Skórka P, Wójcik JD, Martyka R (2005) Colonization and population growth of yellow-legged gull Larus cachinnans in southeastern Poland: causes and influence on native species. Ibis 147:471–482
Southern WE, Southern MD (1984) Herring gulls specialize as ring-billed gull predators. Colon Waterbirds 7:105–110
Spear LB, Pyle P, Nur N (1998) Natal dispersal in the western gull: proximal factors and fitness consequences. J Anim Ecol 67:165–179
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Sydeman WJ, Penniman JF, Penniman TM, Pyle P, Ainley DG (1991) Breeding performance in the western gull: effects of parental age, timing of breeding and year in relation to food availability. J Anim Ecol 60:135–149
Thyen S, Becker PH (2006) Effects of individual life-history traits and weather on reproductive output of black-headed gulls Larus ridibundus breeding in the Wadden Sea, 1991–97. Bird Study 53:132–141
Tinbergen N (1967) Adaptive features of the black-headed gull Larus ridibundus. Proc Int Ornithol Congr 14:43–59
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine, Chicago, pp 139–179
Vidal E, Medail F, Tatoni T (1998) Is the Yellow-legged Gull a superabundant bird species in the Mediterranean? Impact on fauna and flora, conservation measures and research priorities. Biodivers Conserv 7:1013–1026
Wendeln H, Becker PH (1999) Effects of parental quality and effort on the reproduction of common terns. J Anim Ecol 68:205–214
Whittam RM, Leonard ML (2000) Characteristics of predators and offspring influence nest defense by arctic and common terns. Condor 102:301–306
Wiebe KL, Bortolotti GR (2009) Egg size and clutch size in the reproductive investment of American kestrels. J Zool 237:285–301
Wilds C, Czaplak D (1994) Yellow-legged gulls (Larus cachinnans) in North America. Wilson Bull 106:344–356
Wójcik JD, Skórka P, Martyka R (2005) Influence of invasive Caspian gull Larus cachinnans on the native waterbird community. In: Abstract book of the 1st congress of polish ornithologists. Olsztyn, p 198
Yorio P, Quintana F (1997) Predation by Kelp Gulls Larus dominicanus at a mixed-species colony of royal terns Sterna maxima and Cayenne terns Sterna eurygnatha in Patagonia. Ibis 139:536–541
Zielińska M, Zieliński P, Kołodziejczyk P, Szewczyk P, Betleja J (2007) Expansion of the Mediterranean gull Larus melanocephalus in Poland. J Ornithol 148:543–548
Zink AG (2003) Quantifying the costs and benefits of parental care in female treehoppers. Behav Ecol 14:687–693
Acknowledgments
We thank Peter H. Becker, Martti Hario and an anonymous referee for their valuable comments on earlier versions of the manuscript. This study was partially funded by projects NN304 2370 33 and IP 2011 029671 from the Polish Ministry of Science and Higher Education.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. H. Becker.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Skórka, P., Wójcik, J.D., Martyka, R. et al. Numerical and behavioural response of Black-headed Gull Chroicocephalus ridibundus on population growth of the expansive Caspian Gull Larus cachinnans . J Ornithol 153, 947–961 (2012). https://doi.org/10.1007/s10336-012-0824-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0824-4