Abstract
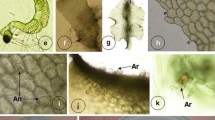
In-vitro studies of the ontogeny and mating system of the gametophytes of Lepisorus nudus were carried out through multispore and isolate cultures lasting 23 weeks. Spore germination begins early, on day 5–6. Spore germination pattern was Vittaria type and the germination percentage reached 82.69% (± 3.20%). Filamentous gametophyte did not branch and never produce separate prothalli. Occasionally the branching and separate prothalli were produced from mature and cordate gametophytes. Prothallial development was Drynaria type (cordate gametophytes with notched apex) contrary to other known species of Lepisorus, where gametophyte development was Kaulinia type (strap gametophytes without apical notch). Gametophyte production in multispore cultures reached up-to 75.6% (± 18.85%). All isolates initially produced archegonia and antheridia only after a prolonged cessation of production in archegonia. In contrast, only 37.2% (±12.63%) of individuals in multispore culture exhibited the same pattern with 29.8% (±7.56%) developing as males that did not produce archegonia by the end of the study. Only 37.2% (±12.63%) of archegoniate gametophytes developed antheridia by the end of the study and only once archegonia had degenerated; i.e., a temporal gap existed in expression of female and male gametangia. In multispore culture, only 26.21% (±5.70%) sporophytes developed on 160th day by fusion of female and male gametes that were derived from matings between sib gametophytes. In contrast, isolated gametophytes did not produce sporophytes. In isolate gametophytes, mature archegonia could not take delivery of male gametangia because antheridia were produced sequentially. This study suggests that the sequential expression of gametangia and absence of the intragametophytic selfing may also be a possible cause of reproductive barriers. Lepisorus nudus promotes inter-gametophytic selfing as an adaptive mechanism for reproductive success in multispore culture. This study presents a detailed account on reproductive biology of the taxa whose population is decreasing at distressing rate.


Similar content being viewed by others
References
Behera SK, Rawat VK, Singh AP, Khare PB (2011) Studies on the spore germination, developmental pattern and sexuality of gametophyte in Dipteris wallichii (R. Br. ex Hook. et Grev.) T. Moore. Indian Fern J 28:172–178
Bir SS, Trikha CK (1974) Taxonomic revision of the Polypodiaceous genera of India-VI. Lepisorus excavatus Group. Am Fern J 64:49–63
Chandra S (2000) The ferns of India (Enumeration, Synonyms and Distribution). International Book Publisher and Distributors, Dehradun p (i–xii) 1–459
Chandra S, Fraser-Jenkins CR, Kumari A, Srivastava A (2008) A summary of the status of threatened Pteridophytes of India. Taiwania 53:170–209
Chiou WL, Farrar DR (1997) Antheridiogen production and response in Polypodiaceae species. Am J Bot 84:633–640
Chiou WL, Farrar DR, Ranker TA (2002) The mating systems of some epiphytic Polypodiaceae. Am Fern J 92:65–79
Crist KC, Farrar DR (1983) Genetic load and long distance dispersal in Asplenium platyneuron. Can J Bot 61:1809–1814
Dixit RD (1984) A census of Indian Pteridophytes. Director, Botanical Survey of India, Howrah, p (i–iii) 1–177
Flinn KM (2006) Reproductive biology of three fern species may contribute to differential colonization success in post agricultural forests. Am J Bot 93:1289–1294
Haig D, Westoby M (1988) Sex expression in homosporus ferns: an evolutionary perspective. Evol Trend Plant 2:111–119
Hassler M, Swale B (2001) Checklist of ferns and fern allies. In: Hassler M, Swale B [com.], pp 1–872. http://homepages.caverock.net.nz/~bj/fern/list.htm. Accessed 28 Mar 2016
Haufler CH, Windham MD, Ranker TA (1990) Biosystematic analysis of the Cystopteris tennesseensis complex. Ann Mo Bot Gard 77:314–329
Hennipman E, Kramer KU, Veldhoen P (1990) Polypodiaceae. In: Kubitzki K (ed) The families and genera of vascular plants. Springer, Berlin, p 203–230
Hooper EA, Haufler CH (1997) Genetic diversity and breeding system in a group of neotropical epiphytic ferns (Pleopeltis; Polypodiaceae). Am J Bot 84:1664–1674
Khullar SP (1994) An illustrated fern flora of West Himalaya (I). International Book Publisher and Distributors, Dehradun, p (i–xxxx) 1–506
Klekowski EJ Jr, Baker HG (1966) Evolutionary significance of polyploidy in the Pteridophyta. Science 153:305–307
Klekowski EJ Jr (1968) Reproductive biology and evolution in the Pteridophyta. Ph. D. dissertation. University of California, Berkeley
Klekowski EJ Jr, Lloyd RM (1968) Reproductive biology of the Pteridophyta, I. General considerations and a study of Onoclea sensibilis L. J Linn Soc (Bot) 60:315–324
Klekowski EJ Jr (1969a) Reproductive biology of the Pteridophyta II, theoretical consideration. Bot J Linn Soc 62:347–359
Klekowski EJ Jr (1969b) Reproductive biology of the Pteridophyta III, a study of Blechnaceae. Bot J Linn Soc 62:361–377
Klekowski EJ Jr (1979) The genetics and reproductive biology of ferns. In: Dyer AF (ed) The experimental biology of ferns, Academic Press, London, pp 133–170
Liu QR, Ming GH, Ge Y, Zhang XC (2008) A taxonomic revision of Lepisorus sect. Hymenophyton Polypodiaceae from China. J Syst Evol 46:906–915
Lloyd RM (1974) Mating systems and genetic load in pioneer and non-pioneer Hawaiian Pteridophyta. Bot J Linn Soc 69:23–35
Masuyama S, Watano Y (1990) Trends for inbreeding in polyploid Pteridophytes. Plant Species Biol 5:13–17
Mitsuta S (1981) Venation of Lepisorus and Pleopeltis: Polypodiaceae. Acta Phytotaxon et Geobot 32:147–164
Naf U, Nakanishi K, Endo M (1975) On the physiology and chemistry of fern antheridiogens. Bot Rev 41:315–359
Nampy S, Madhusoodanan PV (1998) Fern flora of South India - taxonomic revision of Polypodioid ferns. Daya Publishing House, New Delhi
Nayar BK (1961) Studies in Polypodiaceae. VII. Morphology of the gametophyte of Lepisorus excavatus (Bory) Ching. Sci Cult 27:345–347
Nayar BK (1967) Morphology of the spores and prothallus of Christiopteris tricuspis. Am Fern J 57:15–27
Nayar BK, Kaur S (1968) Spore germination in homosporus ferns. J Palynol 4:1–14
Nayar BK, Kaur S (1971) Gametophytes of homosporus ferns. Bot Rev 37:295–396
Nayar BK, Raza F (1970) The prothalli of some Polypodiaceae–II. Lepisorus loriformis, L. thunbergianus, Polypodium vulgare and Weatherbya accedens. J Indian Bot Soc 49:81–86
Negi S, Tewari LM, Pangtey YPS, Kumar S, Martolia A, Jalal J, Upreti K (2009) Taxonomic studies on the family Polypodiaceae (Pteridophyta) of Nainital Uttarakhand. N Y Sci J 2:47–83
Nondorf LS, Dooley AM, Palmieri M, Swatzell J (2003) The effects of pH, temperature, light intensity, light quality, and moisture levels on spore germination in Cheilanthes feei of Southeast Missouri. Am Fern J 93:56–69
Paciencia MLB, Prado J (2004) Efeitos de borda sobre a comunidade de pteridófitas na Mata Atlântica da região de Uma, sul da Bahia, Brasil. Rev Bras de Bot 27:641–653
Prada C, Moreno V, Gabriel JM, Galan Y (2008) Gametophyte development, sex expression and antheridiogen system in Pteris incompleta Cav. (Pteridaceae). Am Fern J 98:14–25
Qi XP, Zhang XC (2009) Taxonomic revision of Lepisorus (J. Sm.) Ching sect. Lepisorus (Polypodiaceae) from China. J Syst Evol 47:581–598
Ranker TA, Houston HA (2002) Is gametophyte sexuality in the laboratory a good predictor of sexuality in nature? Am Fern J 92:112–118
Ranker TA, Gemill CEC, Trapp PG (2000) Microevolutionary pattern and processes of the native Hawaiian colonizing fern Odontosoria chinensis (Lindsaeaceae). Evol Int J Org Evol 54:828–839
Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Magallon S, Lupia R (2004) Ferns diversified in the shadow of angiosperms. Nature 428:553–557
Singh VP, Roy SK (1977) Mating systems and distribution in some tropical ferns. Ann Bot (Lond) 41:1055–1060
Singh AP, Mishra S, Gupta S, Behera SK, Khare PB (2009) Studies on the Genus Ophioglossum L. in Pachmarhi biosphere reserve, Madhya Pradesh-India. Taiwania 54:353–364
Smith AR (1972) Comparison of fern and flowering plant distributions with some evolutionary interpretations for ferns. Biotropica 4:4–9
Soltis DE, Soltis PS (1986) Electrophoretic evidence for inbreeding in the fern Botrychium virginianum (Ophioglossaceae). Am J Bot 74:504–509
Tagawa M, Iwatsuki K (1972) Families and genera of the pteridophytes known from Thailand. Mem Fac Sci Kyoto Univ Ser Biol 5:67–88
Vasudeva SM (1995) Peculiarities of pteridophytic flora of Pachmarhi, Satpura Hills (central India). Indian Fern J 12:29–42
Verma SC (2003) Some aspects of reproductive biology of the gametophyte generation of homosporus ferns. In: Chandra S, Srivastava M (eds) Pteridology in the New Millennium. Kluwer Academic Publishers, Netherland, p 455–484
Wang L, Qi XP, Xiang QP, Heinrichs J, Schneider H, Zhang XC (2010) Phylogeny of the paleotropical fern genus Lepisorus (Polypodiaceae, Polypodiopsida) inferred from four chloroplast DNA regions. Mol Phylogenet Evol 54:211–225
Wang L, Wu ZQ, Bystriakova N, Ansell SW, Xiang QP (2011) Phylogeography of the Sino-Himalayan fern Lepisorus clathratus on ‘‘the roof of the world’’. PLoS One 6:e25896. doi:10.1371/journal.pone.0025896
Zink MJ (1993) Systematics of the fern genus Lepisorus (J. Smith) Ching (Polypodiacea-Lepisoreae). Ph. D. Dissertation Zurich University, Zurich, p 3–30, 55–119
Acknowledgements
Authors are thankful to Director, CSIR-National Botanical Research Institute, Lucknow for providing infrastructural facilities. Thanks are also due to the Council of Scientific & Industrial Research, New Delhi for financial assistance under SIP-005 to support this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.P., Johari, D. & Khare, P.B. Studies on ontogeny and reproductive behaviour of Lepisorus nudus (Hook.) Ching (Polypodiaceae). J Plant Res 130, 281–290 (2017). https://doi.org/10.1007/s10265-016-0891-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0891-3