Abstract
The vertical gradient of the leaf nitrogen content in a plant canopy is one of the determinants of vegetation productivity. The ecological significance of the nitrogen distribution in plant canopies has been discussed in relation to its optimality; nitrogen distribution in actual plant canopies is close to but always less steep than the optimal distribution that maximizes canopy photosynthesis. In this paper, I review the optimality of nitrogen distribution within canopies focusing on recent advancements. Although the optimal nitrogen distribution has been believed to be proportional to the light gradient in the canopy, this rule holds only when diffuse light is considered; the optimal distribution is steeper when the direct light is considered. A recent meta-analysis has shown that the nitrogen gradient is similar between herbaceous and tree canopies when it is expressed as the function of the light gradient. Various hypotheses have been proposed to explain why nitrogen distribution is suboptimal. However, hypotheses explain patterns observed in some specific stands but not in others; there seems to be no general hypothesis that can explain the nitrogen distributions under different conditions. Therefore, how the nitrogen distribution in canopies is determined remains open for future studies; its understanding should contribute to the correct prediction and improvement of plant productivity under changing environments.



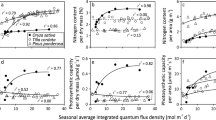
Redrawn from Hikosaka et al. (2016a) with modifications

Redrawn from Hikosaka (2014) with modifications


Redrawn from Hikosaka et al. (2016b) with modifications

Similar content being viewed by others
References
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Anten NPR (2002) Evolutionarily stable leaf area production in plant populations. J Theor Biol 217:15–32
Anten NPR (2005) Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Ann Bot 95:495–506
Anten NPR, Schieving F, Werger MJA (1995a) Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia 101:504–513
Anten NPR, Schieving F, Medina E, Werger MJA, Schuffelen P (1995b) Optimal leaf area indices in C3 and C4 mono- and dicotyledonous species at low and high nitrogen availability. Physiol Plant 95:541–550
Anten NPR, Hernandez R, Medina EM (1996) The photosynthetic capacity and leaf nitrogen concentration as related to light regime in shade leaves of a montane tropical forest tree, Tetrorchidium rubrivenium. Funct Ecol 10:491–500
Anten NPR, Hikosaka K, Hirose T (2000) Nitrogen utilization and the photosynthetic system. In: Marshal B, Roberts J (eds) Leaf development and canopy growth. Sheffield Academic, Sheffield, pp 171–203
Awad H, Barigah T, Badel E, Cochard H, Herbette S (2010) Poplar vulnerability to xylem cavitation acclimates to drier soil conditions. Physiol Plant 139:280–288
Ballaré CL, Scopel AL, Sánchez RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247:329–332
Begg JE, Turner NC (1970) Water potential gradients in field tobacco. Plant Physiol 46:343–346
Buckley TN, Cescatti A, Farquhar GD (2013) What does optimization theory actually predict about crown profiles of photosynthetic capacity when models incorporate greater realism? Plant Cell Environ 36:1547–1563
Charles-Edwards DA, Stutzel H, Ferraris R, Beech DF (1987) An analysis of spatial variation in the nitrogen content of leaves from different horizons within a canopy. Ann Bot 60:421–426
Chen JL, Reynolds JF, Harley PC, Tenhunen JD (1993) Coordination theory of leaf nitrogen distribution in a canopy. Oecologia 93:63–69
Coble AP, Cavaleri MA (2015) Light acclimation optimizes leaf functional traits despite height-related constraints in a canopy shading experiment. Oecologia 177:1131–1143
Connor DJ, Hall AJ, Sadras VO (1993) Effect of nitrogen content on the photosynthetic characteristics of sunflower leaves. Aust J Plant Physiol 20:251–263
de Pury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20:537–557
DeJong TM, Doyle JF (1985) Seasonal relationships between leaf nitrogen content (photosynthetic capacity) and leaf canopy light exposure in peach (Prunus persica). Plant Cell Environ 8:701–706
Dewar RC, Tarvainen L, Parker K, Wallin G, McMurtrie RE (2012) Why does leaf nitrogen decline within tree canopies less rapidly than light? An explanation from optimization subject to a lower bound on leaf mass per area. Tree Physiol 32:520–534
Dreccer MF, Van Oijen M, Schapendonk AHCM, Pot CS, Rabbinge R (2000) Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy: impact on canopy photosynthesis. Ann Bot 86:821–831
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Farquhar GD (1989) Models of integrated photosynthesis of cells and leaves. Philos Trans R Soc Lond B 323:357–367
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Field C (1983) Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Franklin O, Ågren GI (2002) Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Funct Ecol 16:727–733
Gallego HA, Rico M, Moreno G, Santa Regina I (1994) Leaf water potential and stomatal conductance in Quercus pyrenaica Willd. forests: vertical gradients and response to environmental factors. Tree Physiol 14:1039–1047
Givnish TJ (1982) On the adaptive significance of leaf height in forest herbs. Am Nat 120:353–381
Gómez S, Ferrieri RA, Schueller M, Orians CM (2010) Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol 188:835–844
Hellkvist J, Richards GP, Jarvis PG (1974) Vertical gradients of water potential and tissue water relations in Sitka spruce trees measured with the pressure chamber. J Appl Ecol 11:637–668
Hikosaka K (2003) A model of dynamics of leaves and nitrogen in a canopy: an integration of canopy photosynthesis, leaf life-span, and nitrogen-use efficiency. Am Nat 162:149–164
Hikosaka K (2004) Interspecific difference in the photosynthesis-nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hikosaka K (2010) Mechanisms underlying interspecific variation in photosynthetic capacity across wild plant species. Plant Biotech 27:223–229
Hikosaka K (2014) Optimal nitrogen distribution within a leaf canopy under direct and diffuse light. Plant Cell Environ 9:2077–2085
Hikosaka K, Anten NPR (2012) An evolutionary game of leaf dynamics and its consequences on canopy structure. Funct Ecol 26:1024–1032
Hikosaka K, Hirose T (1997) Leaf angle as a strategy for light competition: optimal and evolutionarily stable light-extinction coefficient within a canopy. Écoscience 4:501–507
Hikosaka K, Shigeno A (2009) The role of Rubisco and cell walls in the interspecific variation in photosynthetic capacity. Oecologia 160:443–451
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Hikosaka K, Terashima I, Katoh S (1994) Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading. Oecologia 97:451–457
Hikosaka K, Sudoh S, Hirose T (1999) Light acquisition and use of individuals competing in a dense stand of an annual herb, Xanthium canadense. Oecologia 118:388–396
Hikosaka K, Nagashima H, Harada Y, Hirose T (2001) A simple quantification of interaction between individuals competing for light in a monospecific stand. Funct Ecol 15:642–646
Hikosaka K, Yamano T, Nagashima H, Hirose T (2003) Light-acquisition and use of individuals as influenced by elevated CO2 in even-aged monospecific stands of Chenopodium album. Funct Ecology 17:786–795
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Hikosaka K, Kawauchi Y, Kurosawa T (2010) Why does Viola hondoensis shed their winter leaves in spring? Am J Bot 97:1944–1950
Hikosaka K, Yasumura Y, Muller O, Oguchi R (2014) Resource allocation and trade-offs in carbon gain of leaves under changing environment. In: Tausz M, Grulke N (eds) Trees in a changing environment. Plant ecophysiology, vol 9. Springer, Dordrecht
Hikosaka K, Kumagai T, Ito A (2016a) Modeling canopy photosynthesis. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy photosynthesis: from basics to applications. Springer, Berlin, pp 239–268
Hikosaka K, Anten NPR, Borjigidai A, Kamiyama C, Sakai H, Hasegawa T, Oikawa S, Iio A, Watanabe M, Koike T, Nishina K, Ito A (2016b) A meta-analysis of leaf nitrogen distribution within plant canopies. Ann Bot (in press)
Hirose T (2005) Development of the Monsi–Saeki theory on canopy structure and function. Ann Bot 95:483–494
Hirose T, Werger MJA (1987a) Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiol Plant 70:215–222
Hirose T, Werger MJA (1987b) Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72:520–526
Hollinger DY (1996) Optimality and nitrogen allocation in a tree canopy. Tree Physiol 16:627–634
Kull O, Niinemets U (1998) Distribution of leaf photosynthetic properties in tree canopies: comparison of species with different shade tolerance. Funct Ecol 12:472–479
Lake JA, Quick WP, Beerling DJ, Woodward FI (2001) Signal from mature to new leaves. Nature 411:154
Lemoine D, Cochard H, Granier A (2002) Within crown variation in hydraulic architecture in beech (Fagus sylvatica L): evidence for a stomatal control of xylem embolism. Ann For Sci 59:19–27
Lloyd J, Patino S, Paiva RQ et al (2010) Optimisation of photosynthetic carbon gain and within-canopy gradients of associated foliar traits for Amazon forest trees. Biogeosciences 7:1833–1859
Matsumoto Y, Oikawa S, Yasumura Y, Hirose T, Hikosaka K (2008) Reproductive yield of individuals competing for light in a dense stand of Xanthium canadense. Oecologia 157:185–195
Meir P, Kruijt B, Broadmeadow M, Barbosa E, Kull O, Carswell F, Nobre A, Jarvis PG (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen and leaf mass per unit area. Plant Cell Environ 25:343–357
Mooney HA, Gulmon SL (1979) Environmental and evolutionary constraints on the photosynthetic characteristics of higher plants. In: Solbrig OT, Jain S, Johnson GB, Raven PH (eds) Topics in plant population biology. Columbia University Press, New York, pp 316–337
Mooney HA, Field C, Gulmon SL, Bazzaz FA (1981) Photosynthetic capacities in relation to leaf position in desert versus old field annuals. Oecologia 50:109–112
Moreau D, Allard V, Gaju O, Le Gouis J, Foulkes MJ, Martre P (2012) Acclimation of leaf nitrogen to vertical light gradient at anthesis in wheat is a whole-plant process that scales with the size of the canopy. Plant Physiol 160:1479–1490
Muller O, Hirose T, Werger MJA, Hikosaka K (2011) Optimal use of leaf nitrogen explains seasonal change in leaf nitrogen content of an understory evergreen shrub. Ann Bot 108:529–536
Nagashima H, Hikosaka K (2011) Plants in a crowded stand regulate their height growth so as to maintain similar heights to neighbours even when they have potential advantages in height growth. Ann Bot 108:207–214
Nagashima H, Hikosaka K (2012) Not only light quality but also mechanical stimuli are involved in height convergence in crowded Chenopodium album stands. New Phytol 195:803–811
Niinemets Ü (2012) Optimization of foliage photosynthetic capacity in tree canopies: towards identifying missing constraints. Tree Physiol 32:505–509
Niinemets U, Sonninen E, Tobias M (2004) Canopy gradients in leaf intercellular CO2 mole fractions revisited: interactions between leaf irradiance and water stress need consideration. Plant Cell Environ 27:569–583
Niinemets Ü, Keenan TF, Hallik L (2015) A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol 205:973–993
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Oguchi R, Hikosaka K, Hiura T, Hirose T (2008) Costs and benefits of photosynthetic light acclimation by tree seedlings in response to gap formation. Oecologia 155:665–675
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425
Pearcy RW (1983) The light environment and growth of C3 and C4 tree species in the understory of a Hawaiian forest. Oecologia 58:19–25
Peltoniemi MS, Duursma RA, Medlyn BE (2012) Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiol 32:510–519
Pons TL (2016) Regulation of leaf traits in canopy gradients. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy photosynthesis: from basics to applications. Springer, Berlin, pp 143–168
Pons TL, Schieving F, Hirose T, Werger MJA (1989) Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris. In: Lambes H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequence of variation in growth rate and productivity of higher plants. SPB Academic Publishing, The Hague, pp 175–186
Schieving F, Poorter H (1999) Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytol 143:201–211
Schieving F, Pons TL, Werger MJA, Hirose T (1992) Vertical distribution of nitrogen in photosynthetic activity at different plant densities in Carex acutiformis. Plant Soil 142:9–17
Sellers PJ, Berry JA, Collatz GJ, Field CB, Hall FG (1992) Canopy reflectance, photosynthesis and transpiration. III: a reanalysis using improved leaf models and a new canopy integration scheme. Remote Sens Environ 42:187–216
Shiratsuchi H, Yamagishi T, Ishii R (2006) Leaf nitrogen distribution to maximize the canopy photosynthesis in rice. Field Crop Res 95:291–304
Stockhoff BA (1994) Maximisation of daily canopy photosynthesis: effects of herbivory on optimal nitrogen distribution. J Theor Biol 169:209–220
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Tang YH, Washitani I, Tsuchiya T, Iwaki H (1988) Fluctuation of photosynthetic photon flux density within a Miscanthus sinensis canopy. Ecol Res 3:253–266
Tarvainen L, Wallin G, Räntfors M, Uddling J (2013) Weak vertical canopy gradients of photosynthetic capacities and stomatal responses in a fertile Norway spruce stand. Oecologia 173:1179–1189
Terashima I, Miyazawa SI, Hanba YT (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114:93–105
Terashima I, Araya T, Miyazawa SI, Sone K, Yano S (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Ann Bot 95:507–519
Turnbull TL, Kelly N, Adams MA, Warren CR (2007) Within-canopy nitrogen and photosynthetic gradients are unaffected by soil fertility in field-grown Eucalyptus globulus. Tree Physiol 27:1607–1617
Tuzet A, Perrier A, Leuning R (2003) A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ 26:1097–1116
Tyree MT (2003) Hydraulic limits on tree performance: transpiration, carbon gain and growth of trees. Trees 17:95–100
Tyree MT, Kolb KJ, Rood SB, Patiño S (1994) Vulnerability to drought-induced cavitation of riparian cottonwoods in Alberta: a possible factor in the decline of the ecosystem? Tree Physiol 14:455–466
Warren CR, Adams MA (2001) Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ 24:597–609
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Yano S, Terashima I (2001) Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant Cell Physiol 42:1303–1310
Acknowledgments
I thank Professors Niels Anten and Ülo Niinemets for helpful comments and Professor Tadaki Hirose for providing data on Solidago altissima canopy. The study was supported by KAKENHI (2114009, 25660113, 25440230, 25291095) and CREST, JST, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hikosaka, K. Optimality of nitrogen distribution among leaves in plant canopies. J Plant Res 129, 299–311 (2016). https://doi.org/10.1007/s10265-016-0824-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0824-1