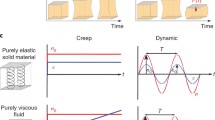
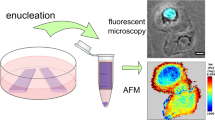
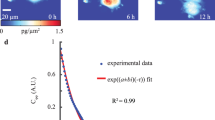
Abstract
A cell’s mechanical properties are important in determining its adhesion, migration, and response to the mechanical properties of its microenvironment and may help explain behavioral differences between normal and cancerous cells. Using fluorescently labeled peroxisomes as microrheological probes, the interior mechanical properties of normal breast cells were compared to a metastatic breast cell line, MDA-MB-231. To estimate the mechanical properties of cell cytoplasms from the motions of their peroxisomes, it was necessary to reduce the contribution of active cytoskeletal motions to peroxisome motion. This was done by treating the cells with blebbistatin, to inhibit myosin II, or with sodium azide and 2-deoxy-\(\textsc {d}\)-glucose, to reduce intracellular ATP. Using either treatment, the peroxisomes exhibited normal diffusion or subdiffusion, and their mean squared displacements (MSDs) showed that the MDA-MB-231 cells were significantly softer than normal cells. For these two cell types, peroxisome MSDs in treated and untreated cells converged at high frequencies, indicating that cytoskeletal structure was not altered by the drug treatment. The MSDs from ATP-depleted cells were analyzed by the generalized Stokes–Einstein relation to estimate the interior viscoelastic modulus \(G^{*}\) and its components, the elastic shear modulus \(G^{\prime }\) and viscous shear modulus \(G^{\prime \prime }\), at angular frequencies between 0.126 and 628 rad/s. These moduli are the material coefficients that enter into stress–strain relations and relaxation times in quantitative mechanical models such as the poroelastic model of the interior regions of cancerous and non-cancerous cells.








Similar content being viewed by others
References
Allingham JS, Smith R, Rayment I (2005) The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12:378–379. doi:10.1038/nsmb908
Atkinson SJ, Hosford MA, Molitoris BA (2004) Mechanism of actin polymerization in cellular ATP depletion. J Biol Chem 279:5194–5199. doi:10.1074/jbc.M306973200
Bertseva E, Grebenkov D, Schmidhauser P et al (2012) Optical trapping microrheology in cultured human cells. Eur Phys J E 35:63. doi:10.1140/epje/i2012-12063-4
Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA (2008) Cytoplasmic diffusion: molecular motors mix it up. J Cell Biol 183:583–587. doi:10.1083/jcb.200806149
Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA (2009) Intracellular transport by active diffusion. Trends Cell Biol 19:423–427. doi:10.1016/j.tcb.2009.04.004
Burnette DT, Shao L, Ott C et al (2014) A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J Cell Biol 205:83–96. doi:10.1083/jcb.201311104
Bursac P, Fabry B, Trepat X et al (2007) Cytoskeleton dynamics: fluctuations within the network. Biochem Biophys Res Commun 355:324–330. doi:10.1016/j.bbrc.2007.01.191
Bursac P, Lenormand G, Fabry B et al (2005) Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater 4:557–561. doi:10.1038/nmat1404
Butcher DT, Alliston T, Weaver VM (2009) A tense situation: forcing tumour progression. Nat Rev Cancer 9:108–122. doi:10.1038/nrc2544
Choi C, Helfman DM (2013) The Ras-ERK pathway modulates cytoskeleton organization, cell motility and lung metastasis signature genes in MDA-MB-231 LM2. Oncogene. doi:10.1038/onc.2013.341
Doi M, Edwards SF (1988) The theory of polymer dynamics. Oxford University Press, Oxford
Elenbaas B, Spirio L, Koerner F et al (2001) Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev 15:50–65. doi:10.1101/gad.828901
Gallet F, Arcizet D, Bohec P, Richert A (2009) Power spectrum of out-of-equilibrium forces in living cells?: amplitude and Frequency Dependence. Soft Matter 5:2947–2953. doi:10.1039/b901311c
Gal N, Weihs D (2012) Intracellular mechanics and activity of breast cancer cells correlate with metastatic potential. Cell Biochem Biophys 63:199–209. doi:10.1007/s12013-012-9356-z
Gal N, Lechtman-Goldstein D, Weihs D (2013) Particle tracking in living cells: a review of the mean square displacement method and beyond. Rheol Acta 52:425–443. doi:10.1007/s00397-013-0694-6
Goldstein D, Elhanan T, Aronovitch M, Weihs D (2013) Origin of active transport in breast-cancer cells. Soft Matter 9:7167–7173. doi:10.1039/C3SM50172H
Grebenkov DS (2011) Probability distribution of the time-averaged mean-square displacement of a Gaussian process. Phys Rev E 84:031124. doi:10.1103/PhysRevE.84.031124
Guo M, Ehrlicher AJ, Jensen MH et al (2014a) Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158:822–832. doi:10.1016/j.cell.2014.06.051
Guo X, Bonin K, Scarpinato K, Guthold M (2014b) The effect of neighboring cells on the stiffness of cancerous and non-cancerous human mammary epithelial cells. New J Phys 16:105002. doi:10.1088/1367-2630/16/10/105002
Guo M, Ehrlicher AJ, Mahammad S et al (2013) The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J 105:1562–1568. doi:10.1016/j.bpj.2013.08.037
Heuser JE, Kirschner MW (1980) Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol 86:212–234
Hoffman BD, Massiera G, Van Citters KM, Crocker JC (2006) The consensus mechanics of cultured mammalian cells. Proc Natl Acad Sci USA 103:10259–10264. doi:10.1073/pnas.0510348103
Ishikawa T, Zhu B-L, Maeda H (2006) Effect of sodium azide on the metabolic activity of cultured fetal cells. Toxicol Ind Health 22:337–341. doi:10.1177/0748233706071737
Kovacs M, Toth J, Hetenyi C et al (2004) Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279:35557–35563. doi:10.1074/jbc.M405319200
Kural C, Kim H, Syed S et al (2005) Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science 308:1469–1472. doi:10.1126/science.1108408
Lee M-H, Wu P-H, Staunton JR et al (2012) Mismatch in mechanical and adhesive properties induces pulsating cancer cell migration in epithelial monolayer. Biophys J 102:2731–2741. doi:10.1016/j.bpj.2012.05.005
Lemmon EW (2014) Thermophysical Properties of Water and Steam. In: Haynes WM (ed) Handb. Chem. Phys. Online, 94th edn. Taylor and Francis, London, pp 6–1. http://www.hbcpnetbase.com. Accessed 29 April 2014
Limouze J, Straight AF, Mitchison T, Sellers JE (2004) Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil 25:337–341. doi:10.1007/s10974-004-6060-7
Li Y, Schnekenburger J, Duits MHG (2009) Intracellular particle tracking as a tool for tumor cell characterization. J Biomed Opt 14:064005. doi:10.1117/1.3257253
Mason TG (2000) Estimating the viscoelastic moduli of complex fluids using the generalized Stokes–Einstein equation. Rheol Acta 39:371–378. doi:10.1007/s003970000094
Mizuno D, Tardin C, Schmidt CF, MacKintosh FC (2007) Nonequilibrium mechanics of active cytoskeletal networks. Science 315:370–373. doi:10.1126/science.1134404
Moeendarbary E, Valon L, Fritzsche M et al (2013) The cytoplasm of living cells behaves as a poroelastic material. Nat Mater 12:253–261. doi:10.1038/NMAT3517
Nagaraja GM, Othman M, Fox BP et al (2006) Gene expression signatures and biomarkers of noninvasive and invasive breast cancer cells: comprehensive profiles by representational difference analysis, microarrays and proteomics. Oncogene 25:2328–2338. doi:10.1038/sj.onc.1209265
Network TPS-OC (2013) A physical sciences network characterization of non-tumorigenic and metastatic cells. Sci Rep. doi:10.1038/srep01449
Paszek MJ, Zahir N, Johnson KR et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254. doi:10.1016/j.ccr.2005.08.010
Pratt JW, Gibbons JD (1981) Concepts of nonparametric theory. Springer, New York
Rapp S, Saffrich R, Jakle U et al (1996) Microtubule-mediated peroxisomal saltations. Ann NY Acad Sci 804:666–668
Saxton MJ (1997) Single-particle tracking: the distribution of diffusion coefficients. Biophys J 72:1744–1753
Schedin P, Keely PJ (2011) Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol 3:a003228. doi:10.1101/cshperspect.a003228
Schrader M, King SJ, Stroh TA, Schroer TA (2000) Real time imaging reveals a peroxisomal reticulum in living cells. J Cell Sci 113:3663–3671
Schrader M, Thiemann M, Fahimi HD (2003) Peroxisomal motility and interaction with microtubules. Microsc Res Tech 61:171–178. doi:10.1002/jemt.10326
Squires TM, Mason TG (2010) Fluid mechanics of microrheology. Annu. Rev. Fluid Mech. Annual Reviews, Palo Alto, pp 413–438
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomater 3:413–438. doi:10.1016/j.actbio.2007.04.002
Van Citters KM, Hoffman BD, Massiera G, Crocker JC (2006) The role of F-actin and myosin in epithelial cell rheology. Biophys J 91:3946–3956. doi:10.1529/biophysj.106.091264
Wick AN, Drury DR, Nakada HI, Wolfe JB (1957) Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 224:963–969
Wiemer E a C, Wenzel T, Deerinck TJ et al (1997) Visualization of the peroxisomal compartment in living mammalian cells: dynamic behavior and association with microtubules. J Cell Biol 136:71–80. doi:10.1083/jcb.136.1.71
Wirtz D (2009) Particle-tracking microrheology of living cells: principles and applications. Annu Rev Biophys 38:301–326. doi:10.1146/annurev.biophys.050708.133724
Zhang B, Zerubia J, Olivo-Marin J-C (2007) Gaussian approximations of fluorescence microscope point-spread function models. Appl Opt 46:1819–1829
Acknowledgments
The authors thank K. Scarpinato and J. Jarzen for providing HMLER cells and insights on their culture, S. Fahrbach for the use of her luminometer for ATP assays, and L. McDonald and G. Marrs for high-resolution confocal fluorescence microscopy images of actin stress fibers in breast epithelial cells. The authors thank D. Lyles for comments on this manuscript. This material is based upon work supported by the National Science Foundation under Grant Number 1106105 (JM, KB, and GH). AMS was supported by National Institutes of Health (T32GM095440) and National Science Foundation (0907738) Grants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smelser, A.M., Macosko, J.C., O’Dell, A.P. et al. Mechanical properties of normal versus cancerous breast cells. Biomech Model Mechanobiol 14, 1335–1347 (2015). https://doi.org/10.1007/s10237-015-0677-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0677-x