Abstract
Background
Treatment of acute (≤3 weeks) acromioclavicular joint dislocation type III–VI is still controversial. Currently, the two modern techniques that are widely used are hook plate (HP) fixation and coracoclavicular ligament fixation using a suspensory loop device that consists of either a tightrope (single or double), endo-button (single or double), or synthetic ligament and absorbable polydioxansulfate sling.
Materials and methods
This systematic review was conducted according to the PRISMA guidelines. Relevant studies that reported Constant-Murley score (CMS), Pain Visual Analog score (VAS) and postoperative complications of either technique were identified from Medline and Scopus from inception to 5 October 2015.
Results
Sixteen studies were included for the analysis of HP fixation, and 25 studies were included for analysis of loop suspensory fixation (LSF). Pooling of mean CMS and VAS scores gave 90.35 (95% CI 87.16, 93.54), 1.51 (95% CI 0.73, 2) in the HP group, and 92.48 (95% CI 90.91, 94.05), 0.32 (95% CI 0, 0.64) in the suspensory loop devices group, respectively. The pooled unstandardized mean differences (UMD) scores of CMS and VAS in LSF were 2.13 (95% CI −1.43, 5.69) and −1.19 (95% CI −2.03, −0.35) when compared to hook plating. The pooled prevalence of LSF and hook plating were 0.08 (95% CI 0.06, 0.10) and 0.05 (95% CI 0.02, 0.08) scores. The chance of having complications in the LSF group was 1.69 (95% CI 1.07, 2.60), which was statistically significantly higher than in the HP group.
Conclusion
LSF have higher shoulder function scores (CMS) and lower postoperative pain when compared to HP fixation; however, there are higher complication rates with LSF when compared to hook plating.
Level of evidence
IV.
Similar content being viewed by others
Introduction
Acromioclavicular (AC) joint (ACJ) dislocation is a common injury in active young adults [5]. The prevalence was approximately 9% of shoulder girdle injuries [3, 28]. AC dislocation is associated with AC and coracoclavicular (CC) ligaments injuries [37]; such injuries are classified into type I–VI on the basis of the radiographic findings using the Rockwood criteria [26, 37]. Non-operative treatment has generally been accepted as the gold standard of treatment in Rockwood I and II lesions [52], whereas the optimal method of treatment for grade III–VI lesions remains a matter of controversy [16, 30, 59]. Although type IV–VI injuries are treated operatively because of their severe instability [45], treatment for type III injury is still controversial [22, 52]. The aim of any surgical approach addressing the instability of the ACJ should be an anatomic reduction and restoration of normal arthrokinetics [18]. Surgical methods employed for the treatment of AC dislocations include extraarticular fixation by CC restoration with metallic cables, autologous ligaments or LARS artificial ligaments, transarticular fixation by hook plate (HP) and Kirschner wires. Kirschner wires and threaded pins are frequently used for temporary fixation of the ACJ. However, serious concerns still exist regarding pin migration or breakage, pin-site infection, fixation failure, and recurrent instability after pin removal [14, 21, 31]. Currently, two modern techniques that are widely used include HP fixation [2, 7, 10, 29, 35, 59] and CC ligament fixation using a suspensory loop device [tightrope (single or double), endo-button (single or double), synthetic ligament and absorbable polydioxansulfate sling (PDS)] [7, 8, 10, 12, 19, 31, 32, 35, 36, 39, 51]. Many case series have reported safe and effective results with the treatment of acute unstable ACJ dislocations by loop suspensory fixation (LSF) [1, 8, 9, 12, 20, 24, 40, 42, 43, 50, 55] and hook plating [7, 17, 25, 38, 39, 49, 56, 57]. More recently, several retrospective cohort studies have aimed to investigate the results of treatment for unstable acute AC dislocations (type III–VI) with HP and LSF. However, there has been no consensus as to which is better for treatment of acute unstable ACJ dislocation. Some studies show benefits of the AC or CC augmentations in pain and Constant-Murley score (CMS) [29, 59], whereas other studies do not [2, 10, 18]. However, LSF consists of variable types of fixation, which include tightrope (single or double), endo-button (single or double), synthetic ligaments and absorbable PDS. Moreover, no high quality methodological quality study [prospective cohort or randomized controlled trials (RCT)] has recently been published. We hypothesized that the impact of each type of fixation with LSF would be comparable to that the HP fixation in acute unstable AC joint dislocation. We therefore conducted a systematic review and meta-analysis that analyzes the available literature, with the aim of comparing the outcomes and safety of LSF, including all different types of implants (tightrope, endo-button, synthetic ligament and PDS) with HP fixation for treatment of acute unstable AC joint dislocation. These clinical outcomes consist of the CMS, Pain Visual Analog score (VAS) and postoperative complications.
Materials and methods
Search strategy
The Medline and Scopus databases were used for identifying relevant studies published in English since the date of inception to 5 October 2015. The PubMed and Scopus search engines were used to locate studies with the following search terms: {[(acromioclavicular joint) OR AC joint] AND [(separation) OR dislocation OR trauma OR injury] AND [(hook plate) OR locking plate OR fixation OR tightrope OR dogbone] AND [(Constant score) OR Constant Murley scale OR CMS OR pain OR UCLA]}. Search strategies for Medline and Scopus are described in detail in the “Appendix 1”. References from the reference lists of included trials and previous systematic reviews were also explored.
Inclusion criteria
Observational studies (e.g., cross-sectional or cohort) that reported clinical outcomes of hook plate or fixation of the CC ligament using an LSF device for treatment of acute unstable ACJ injury were eligible if they met the following criteria:
-
Reported at least one of the following outcomes: CMS, VAS, and postoperative complications.
-
Had sufficient data to extract and pool, i.e., the reported mean, standard deviation (SD), number of subjects according to treatments for continuous outcomes, and number of patients according to treatment for dichotomous outcomes.
The use of a combination of LSF or HP with other methods of fixation and non-English studies were excluded. The reference lists of the retrieved articles were also reviewed to identify publications on the same topic. Where there were multiple publications from the same study group on the same population, the most complete and recent results were used.
Data extraction
Two reviewers (J.K. and A.A.) independently performed data extraction using standardized data extraction forms. General characteristics of the study [i.e., mean age, gender, body mass index (BMI), mean follow up time, mean duration after injury, pain VAS and CMS score at baseline] were extracted. The number of subjects, mean, and SD of continuous outcomes (i.e., VAS and CMS) between groups were extracted. Cross-tabulated frequencies between treatment and all dichotomous outcomes (post-operative complications) were also extracted. Any disagreements were resolved by discussion and consensus with a third party (M.B.).
Outcomes of interest
The outcomes of interests included CMS, VAS, and postoperative complications. These outcomes were measured as reported in the original studies, which were VAS pain scale from 0 to 10 cm (lower values of these scores refer to better outcomes), CMS (0–100, higher values are equivalent to better outcomes). Postoperative complications (wound problems, loss of reduction, implant migration and osteolysis) were considered.
Statistical analysis
For continuous outcomes (CMS and VAS), unstandardized mean differences (UMD) was pooled and calculated using the method as follows [53]: UMD \((d_{I} ) = \bar{x}_{1i} - \bar{x}_{2i} ,\,\text{var} (d_{I} ) = \frac{{{\text{sd}}_{1i}^{2} }}{{n_{1i} }} + \frac{{{\text{sd}}_{2i}^{2} }}{{n_{2i} }},\,w_{I} = \frac{1}{{\text{var} (d_{I} )}},\) where w I is the weighting factor, d I is the standardized/unstandardized difference of means, D I is the pooled difference of means, n 1i and n 2i are the number of subjects in group 1 and 2, n I is n 1i + n 2i, sdI is the pooled SD, var(d I ) is variance of difference, and the subscript I is the study I. Heterogeneity was checked using Q statistic as follows: \(Q = \mathop \sum \nolimits_{i}^{k} w_{i} (d_{i} - D)^{2} ,\;D = \frac{{\mathop \sum \nolimits_{i = 1}^{k} w_{i} d_{i} }}{{\mathop \sum \nolimits_{i = 1}^{k} w_{i} }},\;w_{i} = \frac{1}{{\text{var} (d_{i} )}}.\) The Q statistic follows a Chi square distribution with k − 1 degrees of freedom (df).
For dichotomous outcomes (complications), the prevalence was pooled and calculated using the inverse variance method as follows [53] \(\bar{p} = \frac{{\sum w_{i} p_{i} }}{{\sum w_{i} }}\) where p was the pooled prevalence, p i was the prevalence of complications of each study, w i was 1/var(p i), which was the weight of each study. Heterogeneity of prevalence across studies p was checked as follows: \(\sum w_{i} (p_{i} - \bar{p} )^{2}\). The Q statistic follows a \(\chi^{2}\) distribution with number of studies (k) − 1 degree of freedom (df). The degree of heterogeneity was also quantified using the I 2 statistic [15]. This value can range from 0 to 100%, the closer to 100%, the higher the heterogeneity. If heterogeneity was present, between studies variation was then estimated as follows: \(\tau^{2} = \frac{Q - (k - 1)}{{\sum w_{i} - \frac{{\sum w_{1}^{2} }}{{\sum w_{1} }}}}\) if Q k 1 or 0 otherwise. This was used to calculate a weight term that accounted for variations between studies \(w_{i}^{*} = \frac{1}{{\text{var} (p_{1} ) = \tau^{2} }}\) and then the pooled prevalence was estimated using the random effects model as follows: \(95\% \;{\text{CI}} = \bar{p} ^{*} \pm \frac{1.96}{{\sqrt {\sum w_{i}^{*} } }}.\)
Meta-regression analysis was then applied to explore causes of heterogeneity [15, 54]. Coverable parameters, i.e., type of implants (single and double loops), mean age, percentage male, and type of injuries (III, IV, V and VI) were considered in the meta-regression model. Power of the test for meta-regression was also assessed [44]. The UMD and odds ratio (OR) were estimated by indirect meta-analysis using a random effects model, otherwise a fixed effects model was applied. All analyses were performed using STATA version 14.0 [48].
Results
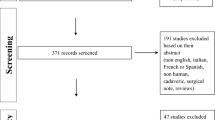
In all, 231 and 387 studies were identified from Medline and Scopus respectively, as described in Fig. 1, of which 49 studies were duplicates, leaving 569 studies for review of titles and abstracts. Of these, 36 articles were relevant and the full papers were retrieved. Characteristics of these studies are described in Table 1: 28 studies were case series reports, 7 were cohort studies and 1 study was a cross-sectional study. Twenty studies reported results of LSF, 11 studies reported results of HP fixation, and 5 studies compared LSF to HP fixation. All 36 studies reported postoperative complications, 25 studies for LSF (22 studies were reported CMS and 12 studies were VAS for pain), and 16 studies for HP fixation (13 studies were reported CMS and 10 studies were VAS for pain). Mean age, percentages of male gender, duration from injury and mean follow up of LSF participants varied from 26 years to 45.6 years, 72.7% to 94.4%, 4.2 days to 13 days and 3 months to 70 months, while HP varied from 29 years to 42.3 years, 84.4% to 100%, 3.5 days to 9.2 days and 3 months to 50.4 months. In all 36 studies, fixation was performed in ACJ injury types III–VI. Twenty-two studies were type III and V, 6 studies were type III, 6 studies were type V, 1 study was type III–IV, 1 study was type IV–V and 1 study was type IV–VI. In the LSF group, 13/24 studies used arthroscopically assisted techniques, as did 4/16 studies in the hook plate group; 12/25 studies used double loop and 13/25 studies used single loop fixation. In the HP group, 14/16 studies reported the time of plate removal, with 8 studies removing the plates within 3 months of initial operation, 4 studies removing the plates at 4 months, and 2 studies removing the plates after 4 months.
Pooled mean CMS in LSF and HP
Thirty-eight studies using LSF and HP fixation in high-grade acute ACJ injury were included for pooling of means and 95% confidence intervals (CI) (Table 2). Among 23 LSF studies, 15 were single bundle fixation, 10 were double bundle fixation, 12 were arthroscopically assisted and 13 were open technique. In terms of CMS, with the LSF group containing 663 patients and HP fixation having 394 patients, the pooled mean CMS of LSF varied greatly across studies (I 2 = 75.2) and was 92.48 (95% CI 90.91, 94.05) (Table 2). The pooled mean of CMS of 16 HP studies varied across studies (I 2 = 85.47) at 90.35 (95% CI 87.16, 93.54). From the result of the indirect meta-analysis, the pooled UMD were 2.13 (95% CI −1.43, 5.69), which translates to the mean CMS of LSF scoring 2.13 higher than HP fixation but the difference was not statistically significant.
Pooled mean VAS in LSF and HP
Ten LSF studies and seven hook plate studies were pooled for VAS pain scores. For the LSF group of 288 patients and HP group of 234 patients, the pooled mean VAS of LSF and HP were homogenous across studies (I 2 = 0 and 15.09), scoring 0.32 (95% CI 0, 0.64) and 1.51 (95% CI 0.73, 2) (Table 2). From indirect meta-analysis, the pooled UMD were −1.19 (95% CI −2.03, −0.35), translating to the mean VAS of LSF being about 1.2 scores statistically significantly lower when compared to hook plating.
Pooled prevalence of LSF and HP
Twenty-five LSF studies and 16 hook plate studies pooled the prevalence of complications after fixation. For the LSF group of 701 patients and HP group of 668 patients, the pooled prevalence of LSF and HP had mild to moderate degrees of heterogeneity across studies (I 2 = 24.27 and 42.13), with scores of 0.08 (95% CI 0.06, 0.10) and 0.05 (95% CI 0.02, 0.08) (Table 3). From indirect meta-analysis, the difference in the risk of having complications between two groups was 1.69 (95% CI 1.07, 2.60), indicating that the chance of having a wound problems, loss of reduction, implant migration and osteolysis in the LSF group was about 1.7 times statistically significant higher than in the HP group (Table 3).
Sources of heterogeneity
Meta-regression was applied to explore the cause of heterogeneity by fitting a co-variable (i.e., age, percentage of sex, type of AC injury, approach, number of bundle, time of plate removal, duration before surgery and type of studies level), and meta-regression was applied to assess this. None of the co-variables could explain the heterogeneity. However, the type of approaches and number of bundles of fixation might be the source of heterogeneity. Therefore, subgroup analyses were performed as described in Table 4.
Discussion
From the current available evidence, this systematic review and meta-analysis has shown the following: LSF implants have higher shoulder function and lower shoulder pain reported by CMS and VAS scores of 2.2 and 1.2 points, respectively, when compared to HP fixation. However, LSF displayed a higher complication rate after surgery, (wound problems, loss of reduction, implant migration and osteolysis) being 1.7 times higher than HP fixation in acute unstable ACJ injury.
Of the previously published studies [2, 10, 18, 29, 59], there have been no high quality studies comparing the results of these two fixation methods. Although there are comparative studies reporting results between the two groups, there is still no clear consensus as to which is preferable. From this study, we have additional evidence that LSF displays a higher improvement of CMS and VAS scores when compared to HP fixation. However, LSF has a higher risk of postoperative complications when compared to HP fixation. The mean CMS, VAS Pain score and prevalence of complications among included studies was heterogeneous, possibly due to methodological and clinical differences. Attempts were made to explore sources of heterogeneity by considering clinical (i.e., age, percentage of sex, type of AC injury, approach, number of bundle, time of plate removal and duration before surgery) and methodological variables (i.e., type of study) in the meta-regression model. None of the co-variables could explain the heterogeneity. However, the degree of heterogeneity did not decrease after pooling all subgroups, indicating the presence of other sources of heterogeneity. There are several important clinical factors that may have had an effect on the results, including the use of two different approaches (arthroscopic or open) and two different implant designs (single or double) that are suspected to be the source of heterogeneity of the LSF devices. Although LSF shows higher complication rates postoperatively, HP fixation is a double procedure that also requires a second surgery for plate removal. After subgroup analyses, the results show that there are still no statistically significant differences in CMS, VAS and complications between different approaches and the number of bundle fixation (Table 4). The timing of the second operation for plate removal displays no statistically significant difference for CMS and VAS outcomes comparing before 3 months and after 3 months of the initial surgery.
Although we were unable to find the source of heterogeneity by meta-regression and subgroup analysis (two different approaches, two different implant designs of LSF devices and the plate removal time after hook plating), several factors must be considered in clinical implementation. Firstly, there is either single [1, 2, 4, 6, 9, 10, 12, 20, 29, 34, 38, 43, 46, 47, 50] or double (V anatomic shape [24] with parallel [8, 18, 33, 40, 42, 55, 58, 59]) bundle LSF fixation, in which double bundles demonstrate higher function scores (CMS) and lower pain VAS. Within the double bundle groups, no significant differences regarding clinical or radiologic results have been found [24]. Secondly, arthroscopic techniques have recently been described in the treatment of acute AC dislocation [1, 9, 18, 29, 34, 40, 42, 43, 46, 50, 55, 59]. With the use of an arthroscopic approach, LSF seems to have higher pain VAS when compared to open LSF [2, 4, 6, 8, 10, 12, 20, 24, 33, 38, 47, 58], but shows no differences in terms of function, outcomes or complication rates. For HP fixation, most studies used an open approach [7, 13, 17, 21, 23, 39, 41, 49, 56], with only one study using an arthroscopic approach [11]. However, after subgroup analysis, there were no differences in pain function and complication outcomes. As for the time of implant removal after initial surgery in the hook plating group, pain VAS was lower when the plate was removed within 3 months [2, 7, 18, 21, 23, 29, 39, 59], while the CMS function score was higher when the plate was removed after 3 months [10, 17, 25, 41, 49, 56] (Table 4). Therefore, the recommended time to remove the implant will depend on the symptoms of each individual patient. If there is persistent pain after surgery, the HP should be removed before 3 months. If the patient has no pain but limited shoulder function; the implant should not be removed prior to 3 months postoperatively.
The strength of this study is that it has a high power to detect a clinical difference between two implant fixations (the minimal clinically important difference of VAS is 1.2 points), with a power of 100% to detect this margin, and a type I error of 1%. This study uses adequate methodology of systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27], as well as providing exploration and reduction of the heterogeneity of the studies using subgroup analysis and adequate statistical analysis.
There were some limitations in this study. Firstly, the quality of studies for the meta-analysis was not high. Ideal evidence for systematic review is an RCT, which is most commonly used in testing the efficacy of surgery. Only 5 trials included were comparative studies (retrospective cohort studies) while 31 trials included were case series reports. This could be a possible source of bias between groups due to the opportunity for selection and different baseline characteristics. Secondly, heterogeneity remains an important factor to be considered in the conduct and interpretation of meta-analysis, and the heterogeneity between studies was great. We applied the random effects meta-analysis to adjust for the differences between studies, and the possible causes of heterogeneity were explored if covariate data at baseline (e.g., age, percentage of sex, type of AC injury, approach, number of bundle, timing of plate removal, duration before surgery and type of studies) were available. The third limitation is that indirect meta-analysis was used for calculating the mean difference and odds ratio between the two groups, due to the fact that most included studies were case series reports of only one technique. The fourth limitation is that there are other outcomes of interest that can be used to compare LSF and HP fixation such as operation cost or post-operative satisfaction and quality of life. However, these factors could not be analyzed because of insufficient data. The last limitation is that most studies had a mean follow up time of approximately 1–2 years; therefore mid-term to long-term effects of the different types of fixation are still unknown.
In summary, for acute high-grade ACJ injuries, both HP and LSF had acceptable post-operative outcomes. LSF provided better postoperative shoulder function (CMS) when compared to HP fixation, but the difference was not statistically significant. LSF provided clinically and statistically significant lower pain VAS when compared to HP fixation. However, LSF had higher complication rates when compared to the HP fixation group. This study shows that both options have advantages and disadvantages and should be chosen based on patient status. In the future, prospective randomized controlled studies are needed to confirm these findings as the current literature is still insufficient.
References
Acar MA, Güleç A, Erkoçak OF, Yilmaz G, Durgut F, Elmadağ M (2015) Percutaneous double-button fixation method for treatment of acute type III acromioclavicular joint dislocation. Acta Orthop Traumatol Turc 49(3):241–248. doi:10.3944/AOTT.2015.14.0230
Andreani L, Bonicoli E, Parchi P, Piolanti N, Michele L (2014) Acromio-clavicular repair using two different techniques. Eur J Orthop Surg Traumatol 24(2):237–242. doi:10.1007/s00590-013-1186-1
Babhulkar A, Pawaskar A (2014) Acromioclavicular joint dislocations. Curr Rev Musculoskelet Med 7(1):33–39. doi:10.1007/s12178-013-9199-2
Beris A, Lykissas M, Kostas-Agnantis I, Vekris M, Mitsionis G, Korompilias A (2013) Management of acute acromioclavicular joint dislocation with a double-button fixation system. Injury 44(3):288–292. doi:10.1016/j.injury.2013.01.002
Bishop JY, Kaeding C (2006) Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev 14(4):237–245. doi:10.1097/01.jsa.0000212330.32969.6e
Choi SW, Lee TJ, Moon KH, Cho KJ, Lee SY (2008) Minimally invasive coracoclavicular stabilization with suture anchors for acute acromioclavicular dislocation. Am J Sports Med 36(5):961–965. doi:10.1177/0363546507312643
Di Francesco A, Zoccali C, Colafarina O, Pizzoferrato R, Flamini S (2012) The use of hook plate in type III and V acromio-clavicular Rockwood dislocations: clinical and radiological midterm results and MRI evaluation in 42 patients. Injury 43(2):147–152. doi:10.1016/j.injury.2011.04.002
Dimakopoulos P, Panagopoulos A, Syggelos SA, Panagiotopoulos E, Lambiris E (2006) Double-loop suture repair for acute acromioclavicular joint disruption. Am J Sports Med 34(7):1112–1119. doi:10.1177/0363546505284187
El Sallakh SA (2012) Evaluation of arthroscopic stabilization of acute acromioclavicular joint dislocation using the TightRope system. Orthopedics 35(1):e18–e22. doi:10.3928/01477447-20111122-13
Eschler A, Gradl G, Gierer P, Mittlmeier T, Beck M (2012) Hook plate fixation for acromioclavicular joint separations restores coracoclavicular distance more accurately than PDS augmentation, however presents with a high rate of acromial osteolysis. Arch Orthop Trauma Surg 132(1):33–39. doi:10.1007/s00402-011-1399-x
Gille J, Heinrichs G, Unger A, Riepenhof H, Herzog J, Kienast B, Oheim R (2013) Arthroscopic-assisted hook plate fixation for acromioclavicular joint dislocation. Int Orthop 37(1):77–82. doi:10.1007/s00264-012-1691-6
Greiner S, Braunsdorf J, Perka C, Herrmann S, Scheffler S (2009) Mid to long-term results of open acromioclavicular-joint reconstruction using polydioxansulfate cerclage augmentation. Arch Orthop Trauma Surg 129(6):735–740. doi:10.1007/s00402-008-0688-5
Guizzi P, Quadri V, Claudio M, Moretta M (2012) Rockwood type iii acromioclavicular dislocation: clinical and x ray review after treatment with clavicle Hook Plate. Minerva Ortop Traumatol 63(4):271–278
Guttmann D, Paksima NE, Zuckerman JD (2000) Complications of treatment of complete acromioclavicular joint dislocations. Instr Course Lect 49:407–413
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Horst K, Dienstknecht T, Pishnamaz M, Sellei RM, Kobbe P, Pape HC (2013) Operative treatment of acute acromioclavicular joint injuries graded Rockwood III and IV: risks and benefits in tight rope technique vs. k-wire fixation. Patient Saf Surg 7:18. doi:10.1186/1754-9493-7-18
Jafary D, Keihan Shokouh H, Najd Mazhar F, Shariat Zadeh H, Mochtary T (2014) Clinical and radiological results of fixation of acromioclavicular joint dislocation by hook plates retained for more than five months. Trauma Mon 19(2):e13728. doi:10.5812/traumamon.13728
Jensen G, Katthagen JC, Alvarado LE, Lill H, Voigt C (2014) Has the arthroscopically assisted reduction of acute AC joint separations with the double tight-rope technique advantages over the clavicular hook plate fixation? Knee Surg Sports Traumatol Arthrosc 22(2):422–430. doi:10.1007/s00167-012-2270-5
Jobe FW, Bradley JP (1989) The diagnosis and nonoperative treatment of shoulder injuries in athletes. Clin Sports Med 8(3):419–438
Katsenis DL, Stamoulis D, Begkas D, Tsamados S (2015) Minimally invasive reconstruction of acute type IV and Type V acromioclavicular separations. Orthopedics 38(4):e324–e330. doi:10.3928/01477447-20150402-62
Kienast B, Thietje R, Queitsch C, Gille J, Schulz AP, Meiners J (2011) Mid-term results after operative treatment of rockwood grade III–V acromioclavicular joint dislocations with an AC-hook-plate. Eur J Med Res 16(2):52–56
Kim S, Blank A (2014) Management of type 3 acromioclavicular joint dislocations—current controversies. Bull Hosp Jt Dis (2013) 72(1):53–60
Koukakis A, Manouras A, Apostolou CD, Lagoudianakis E, Papadima A, Triantafillou C, Korres D, Allen PW, Amini A (2008) Results using the AO hook plate for dislocations of the acromioclavicular joint. Expert Rev Med Devices 5(5):567–572. doi:10.1586/17434440.5.5.567
Kraus N, Haas NP, Scheibel M, Gerhardt C (2013) Arthroscopically assisted stabilization of acute high-grade acromioclavicular joint separations in a coracoclavicular Double-TightRope technique: V-shaped versus parallel drill hole orientation. Arch Orthop Trauma Surg 133(10):1431–1440. doi:10.1007/s00402-013-1804-8
Kumar N, Sharma V (2015) Hook plate fixation for acute acromioclavicular dislocations without coracoclavicular ligament reconstruction: a functional outcome study in military personnel. Strateg Trauma Limb Reconstr (Online) 10(2):79–85. doi:10.1007/s11751-015-0228-0
Li Q, Hsueh PL, Chen YF (2014) Coracoclavicular ligament reconstruction: a systematic review and a biomechanical study of a triple endobutton technique. Medicine 93(28):e193. doi:10.1097/md.0000000000000193
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100
Macdonald PB, Lapointe P (2008) Acromioclavicular and sternoclavicular joint injuries. Orthop Clin N Am 39(4):535–545. doi:10.1016/j.ocl.2008.05.003
Metzlaff S, Rosslenbroich S, Forkel PH, Schliemann B, Arshad H, Raschke M, Petersen W (2016) Surgical treatment of acute acromioclavicular joint dislocations: hook plate versus minimally invasive reconstruction. Knee Surg Sports Traumatol Arthrosc 24(6):1972–1978. doi:10.1007/s00167-014-3294-9
Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ (2013) Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J 95-B(12):1595–1602. doi:10.1302/0301-620x.95b12.31802
Morrison DS, Lemos MJ (1995) Acromioclavicular separation. Reconstruction using synthetic loop augmentation. Am J Sports Med 23(1):105–110
Motamedi AR, Blevins FT, Willis MC, McNally TP, Shahinpoor M (2000) Biomechanics of the coracoclavicular ligament complex and augmentations used in its repair and reconstruction. Am J Sports Med 28(3):380–384
Murena L, Vulcano E, Ratti C, Cecconello L, Rolla PR, Surace MF (2009) Arthroscopic treatment of acute acromioclavicular joint dislocation with double flip button. Knee Surg Sports Traumatol Arthrosc 17(12):1511–1515. doi:10.1007/s00167-009-0838-5
Pan Z, Zhang H, Sun C, Qu L, Cui Y (2015) Arthroscopy-assisted reconstruction of coracoclavicular ligament by Endobutton fixation for treatment of acromioclavicular joint dislocation. Arch Orthop Trauma Surg 135(1):9–16. doi:10.1007/s00402-014-2117-2
Patzer T, Clauss C, Kuhne CA, Ziring E, Efe T, Ruchholtz S, Mann D (2013) Arthroscopically assisted reduction of acute acromioclavicular joint separations: comparison of clinical and radiological results of single versus double TightRope technique. Der Unfallchirurg 116(5):442–450. doi:10.1007/s00113-011-2135-2
Petersen W, Wellmann M, Rosslenbroich S, Zantop T (2010) Minimally invasive acromioclavicular joint reconstruction (MINAR). Oper Orthop Traumatol 22(1):52–61. doi:10.1007/s00064-010-3004-4
Rieser GR, Edwards K, Gould GC, Markert RJ, Goswami T, Rubino LJ (2013) Distal-third clavicle fracture fixation: a biomechanical evaluation of fixation. J Shoulder Elb Surg 22(6):848–855. doi:10.1016/j.jse.2012.08.022
Ryhanen J, Leminen A, Jamsa T, Tuukkanen J, Pramila A, Raatikainen T (2006) A novel treatment of grade III acromioclavicular joint dislocations with a C-hook implant. Arch Orthop Trauma Surg 126(1):22–27. doi:10.1007/s00402-005-0074-5
Salem KH, Schmelz A (2009) Treatment of Tossy III acromioclavicular joint injuries using hook plates and ligament suture. J Orthop Trauma 23(8):565–569. doi:10.1097/BOT.0b013e3181971b38
Salzmann GM, Walz L, Buchmann S, Glabgly P, Venjakob A, Imhoff AB (2010) Arthroscopically assisted 2-bundle anatomical reduction of acute acromioclavicular joint separations. Am J Sports Med 38(6):1179–1187. doi:10.1177/0363546509355645
Sarrafan N, Nasab SAM, Gholaminejad S (2012) Comparison of the efficacy of hook plate versus pinning in treatment of acute acromioclavicular joint dislocation. Pak J Med Sci 28(5):879–882
Scheibel M, Dröschel S, Gerhardt C, Kraus N (2011) Arthroscopically assisted stabilization of acute high-grade acromioclavicular joint separations. Am J Sports Med 39(7):1507–1516. doi:10.1177/0363546511399379
Shin SJ, Kim NK (2015) Complications after arthroscopic coracoclavicular reconstruction using a single adjustable-loop-length suspensory fixation device in acute acromioclavicular joint dislocation. Arthrosc J Arthrosc Relat Surg 31(5):816–824. doi:10.1016/j.arthro.2014.11.013
Simmonds MC, Higgins JP (2007) Covariate heterogeneity in meta-analysis: criteria for deciding between meta-regression and individual patient data. Stat Med 26(15):2982–2999. doi:10.1002/sim.2768
Smith TO, Chester R, Pearse EO, Hing CB (2011) Operative versus non-operative management following Rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol 12(1):19–27. doi:10.1007/s10195-011-0127-1
Spoliti M, De Cupis M, Via AG, Oliva F (2014) All arthroscopic stabilization of acute acromioclavicular joint dislocation with fiberwire and endobutton system. Muscles Ligaments Tendons J 4(4):398–403
Stam L, Dawson I (1991) Complete acromioclavicular dislocations: treatment with a Dacron ligament. Injury 22(3):173–176
StataCorp. (2015) Stata statistical software: release 14. StataCorp LP, College Station
Steinbacher G, Sallent A, Seijas R, Boffa JM, Espinosa W, Cugat R (2014) Clavicular hook plate for grade-III acromioclavicular dislocation. J Orthop Surg (Hong Kong) 22(3):329–332
Struhl S, Wolfson TS (2015) Continuous loop double endobutton reconstruction for acromioclavicular joint dislocation. Am J Sports Med 43(10):2437–2444. doi:10.1177/0363546515596409
Taft TN, Wilson FC, Oglesby JW (1987) Dislocation of the acromioclavicular joint. An end-result study. J Bone Joint Surg Am 69(7):1045–1051
Tauber M (2013) Management of acute acromioclavicular joint dislocations: current concepts. Arch Orthop Trauma Surg 133(7):985–995. doi:10.1007/s00402-013-1748-z
Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, Duffy D, Thompson J, Hall I, Kaufman J, Leung TF, Helms PJ, Hakonarson H, Halpi E, Navon R, Attia J (2005) Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 162(3):201–211. doi:10.1093/aje/kwi184
Thompson SG, Higgins JP (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21(11):1559–1573. doi:10.1002/sim.1187
Venjakob AJ, Salzmann GM, Gabel F, Buchmann S, Walz L, Spang JT, Vogt S, Imhoff AB (2013) Arthroscopically assisted 2-bundle anatomic reduction of acute acromioclavicular joint separations: 58-month findings. Am J Sports Med 41(3):615–621. doi:10.1177/0363546512473438
von Heideken J, Bostrom Windhamre H, Une-Larsson V, Ekelund A (2013) Acute surgical treatment of acromioclavicular dislocation type V with a hook plate: superiority to late reconstruction. J Shoulder Elbow Surg 22(1):9–17. doi:10.1016/j.jse.2012.03.003
Wang Y, Zhang J (2014) Acromioclavicular joint reconstruction by coracoid process transfer augmented with hook plate. Injury 45(6):949–954. doi:10.1016/j.injury.2013.12.013
Wei HF, Chen YF, Zeng BF, Zhang CQ, Chai YM, Wang HM, Lu Y (2011) Triple endobuttton technique for the treatment of acute complete acromioclavicular joint dislocations: preliminary results. Int Orthop 35(4):555–559. doi:10.1007/s00264-010-1057-x
Yoon JP, Lee BJ, Nam SJ, Chung SW, Jeong WJ, Min WK, Oh JH (2015) Comparison of results between hook plate fixation and ligament reconstruction for acute unstable acromioclavicular joint dislocation. Clin Orthop Surg 7(1):97–103. doi:10.4055/cios.2015.7.1.97
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interests.
Patient consent
For this type of study informed consent is not required.
Ethical approval
Not applicable as no new patients were involved in this research.
Funding
None.
Appendix
Appendix
Search term and strategy
#1 acromioclavicular joint
#2 AC joint
#3 #1 OR #2
#4 separation
#5 dislocation
#6 trauma
#7 injury
#8 #4 OR #5 OR #6 OR #7
#9 hook plate
#10 locking plate
#11 fixation
#12 tightrope
#13 dogbone
#14 #9 OR #10 OR #11 OR #12 OR #13
#15 Constant score
#16 Constant Murley scale
#17 CMS
#18 pain
#19 UCLA
#20 #15 OR #16 OR #17 OR #18 OR #19
#21 #3 AND #8 AND #14 AND #20
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arirachakaran, A., Boonard, M., Piyapittayanun, P. et al. Post-operative outcomes and complications of suspensory loop fixation device versus hook plate in acute unstable acromioclavicular joint dislocation: a systematic review and meta-analysis. J Orthop Traumatol 18, 293–304 (2017). https://doi.org/10.1007/s10195-017-0451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10195-017-0451-1