Abstract
Background
The RIFLE classification is widely used to assess the severity of acute kidney injury (AKI), but its application to geriatric AKI patients complicated by medical problems has not been reported.
Methods
We investigated 256 geriatric patients (≥65 years old; mean age, 74.4 ± 6.3 years) who developed AKI in the intensive care unit (ICU) according to the RIFLE classification. Etiologic, clinical, and prognostic variables were analyzed.
Results
They were categorized into RIFLE-R (n = 53), RIFLE-I (n = 102), and RIFLE-F (n = 101) groups. The overall in-hospital mortality was 39.8 %. There were no significant differences in RIFLE category between survivors and non-survivors. Survivors had significantly less needs for a ventilator and vasopressor, and lower number of failing organs. Survivors had higher systolic blood pressure, hemoglobin level, and serum albumin levels. We performed a logistic regression analysis to identify the independent predictors of in-hospital mortality. In a univariate analysis, hypertension, chronic kidney disease, RIFLE classification, number of failing organs, need for a ventilator and vasopressor, systolic blood pressure, hemoglobin level, and serum albumin levels were identified as prognostic factors of in-hospital mortality. However, in a multivariate analysis, hypertension, chronic kidney disease, number of failing organs, and serum albumin levels were independent risk factors, with no significant difference for in-hospital mortality with the RIFLE classification.
Conclusion
The RIFLE classification might not be associated with mortality in geriatric AKI patients in the ICU. In geriatric patients with AKI, various factors besides severity of AKI should be considered to predict mortality.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI), which leads to an increase in serum creatinine or a decline in urine output, is associated with significant morbidity and mortality for patients in the intensive care unit (ICU), across the course of hospitalization and for years after hospital discharge [1]. As structural and functional changes associated with aging occur [2], geriatric patients have a tenfold increase in the incidence of AKI, significantly lower rates of renal recovery, and a higher risk of dialysis dependence when compared with younger patients [3]. In addition to this intrinsic renal decline, multiple comorbidities, polypharmacy, and susceptibility to nephrotoxic agents may predispose geriatric patients to the subsequent development of AKI [2, 4].
Research on AKI has long been hampered by inconsistent definitions (as many as 35 different definitions) [5]. In 2004, the Acute Dialysis Quality Initiative (ADQI) published the risk, injury, failure, loss, end-stage (RIFLE) criteria, defining AKI in terms of changes in serum creatinine level from baseline and in urine output [6]. The acronym RIFLE represents three grades of increasing severity of risk, injury, and failure, and two outcome variables, namely loss and end-stage kidney disease. The severity grades R–F are defined based on changes in serum creatinine level or urine output, wherein the worst of each criterion is applied. The two outcome criteria, L and E, are defined by duration of renal function loss.
Previous studies have found that RIFLE classification was useful for predicting renal function recovery, the requirement for renal replacement therapy, length of hospital stay for survivors, and in-hospital mortality in the general population [7]. Additionally, in ICU patients, each RIFLE category was independently associated with hospital mortality after adjusting for multiple covariates in patients with AKI [8]. A recent study of geriatric postoperative AKI patients (≥65 years old), reported that the RIFLE classification was significantly associated with increased hospital mortality [9]. However, this study only included patients with postoperative AKI in the ICU. The disease course of AKI complicated by medical problems may be different with postoperative AKI regards with comorbidities or general patterns of patient’s admission. Application of the RIFLE classification to geriatric AKI patients complicated by medical problems has not been reported in previous studies. The aim of this study was to delineate the clinical features of geriatric patients (defined as age ≥65 years) who developed AKI in the ICU complicated by medical problems and, in particular, to investigate the role of RIFLE classification as a prognostic factor for survival.
Materials and methods
Patients
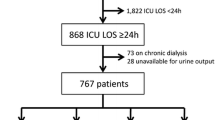
Patients were eligible for enrollment if they were ≥65 years of age and developed AKI in the ICU between January 2009 and April 2013. All the patients were admitted to the ICU because of medical problems. We excluded any patients who were on maintenance dialysis (>3 months of renal replacement therapy) and who stayed in the ICU for less than 48 h. This study protocol was approved by the institutional review board (IRB) of Pusan National University Hospital, Busan, Republic of Korea (E-2014117). Informed consent was waived by the IRB.
Methods
We recorded confidential information on age, sex, comorbidities, possible etiologies for the AKI, duration of ICU stay and hospitalization, and number of failing organs. We also recorded treatments administered during the ICU stay, including ventilator, vasopressor, and dialysis treatments. We investigated the systolic blood pressure, diastolic blood pressure, and laboratory findings at the time of ICU admission.
AKI was defined according to the sRIFLE criteria, in which only serum creatinine levels are used to classify patients. Urine output criteria could not be applied because data regarding urine output amount were not available for the patients. Baseline serum creatinine level was derived from the nadir value recorded within 1 year prior to the index admission. If a baseline serum creatinine level was unavailable, we estimated baseline serum creatinine level obtained by solving the Modification of Diet in Renal Disease (MDRD) study equation, with the assumption of a baseline estimated glomerular filtration rate of 75 mL/min per 1.73 m2 (n = 23) [9]. Comorbidities observed in the patients included diabetes mellitus, hypertension, heart failure, coronary artery disease, atrial fibrillation, chronic obstructive pulmonary disease, liver cirrhosis, hepatitis, malignancy, and chronic kidney disease (CKD). Diabetes mellitus was defined as the use of any hypoglycemic agents. Hypertension was defined as the previous use of antihypertensive agents or blood pressure higher than 140/90 mmHg at admission. Heart failure was defined as the previous diagnosis or positive echocardiographic findings. Coronary artery disease was defined according to previous coronary angiography or compatible electrocardiographic findings. Atrial fibrillation was defined as more than 2 previous episodes of electrocardiographic evidence of atrial fibrillation. Chronic obstructive pulmonary disease was defined as the previous diagnosis or was documented by certified pulmonologists. Liver cirrhosis was identified using compatible image findings. Hepatitis was defined as abnormal liver function with serologic evidence of hepatitis B or C. CKD was defined as a baseline estimated GFR of <60 ml/min per 1.73 m2. Possible etiologies for the AKI included dehydration, infection, bleeding, contrast agents, drug, obstruction, cardiogenic shock, renal infarction, rhabdomyolysis, and hepatorenal syndrome. Number of failing organs were recorded at the time of ICU admission as a severity score (Table 1) [10]. We compared the clinical and biochemical variables in survivors and non-survivors and analyzed in-hospital mortality as a primary end point.
Statistical analyses
Statistical analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation values and compared by using the Student’s t test. Categorical variables were expressed as percentages and analyzed by using the Chi square test. If there were more than two groups, one-way analysis of variance was used for trend analysis. We performed a logistic regression analysis using the entry method to determine the independent predictors of in-hospital mortality. All variables with a P < 0.05 in the univariate analysis were selected and entered into multivariate models. All results were considered statistically significant at P < 0.05.
Results
Demographic and clinical characteristics of the study participants
A total of 256 geriatric patients with AKI in the ICU were enrolled in the study (mean age 74.4 ± 6.3 years, 147 male [57.4 %]) and divided into 3 study subgroups, namely RIFLE-R (n = 53), RIFLE-I (n = 102), and RIFLE-F (n = 101). The baseline characteristics of the study participants are shown in Table 2. There were no significant differences in age and sex between the three groups. Over half of the patients had underlying hypertension (57.0 %), and 38.3 % had been diagnosed with diabetes mellitus. There was a higher incidence of CKD (RIFLE-R vs. RIFLE-I vs. RIFLE-F, 11.3 vs. 17.6 vs. 34.7 %; P = 0.001) in the geriatric patients who developed RIFLE-F AKI. With the exception of CKD, there was no significant difference in comorbidities between the three groups. The most common etiology of AKI was infection (45.7 %), with the second most common etiology being cardiogenic shock (17.2 %). There was no significant difference in hospitalization duration, ICU stay duration, and number of failing organs between the three groups. Of the 256 AKI patients, the overall in-hospital mortality rate was 39.8 %. Although not statistically significant, a lower mortality rate was observed in the RIFLE-R group in comparison to both the RIFLE-I and RIFLE-F groups. The RIFLE-F group received significantly more dialysis treatments than the RIFLE-R and RIFLE-I groups (RIFLE-R vs. RIFLE-I vs. RIFLE-F, 28.3 vs. 26.5 vs. 52.5 %; P < 0.001). Baseline serum creatinine levels increased progressively from the RIFLE-R to the RIFLE-F group (P = 0.008).
Comparisons between the survivors and non-survivors among the geriatric acute kidney injury patients in the ICU
There were no significant differences in age and sex between the survivors and non-survivors (Table 3). The survivors were more likely to have hypertension (64.3 vs. 46.1 %; P = 0.003) and less likely to have CKD (15.6 vs. 34.3 %; P < 0.001). There were no significant differences in RIFLE classification between the survivors and non-survivors. The survivors had significantly less interventions, including ventilator (37.0 vs. 66.7 %, P < 0.001) and vasopressor (54.5 vs. 84.3 %, P < 0.001), along with a lower number of failing organs (1.0 ± 1.0 vs. 1.4 ± 1.0, P < 0.001). The survivors had higher systolic blood pressures at ICU admission (120.7 ± 27.9 vs. 112.9 ± 29.9 mmHg, P = 0.034), hemoglobin levels (11.0 ± 2.2 vs. 10.0 ± 2.1 mg/dL, P = 0.001), and serum albumin levels (3.1 ± 0.7 vs. 2.9 ± 0.6 g/dL, P = 0.003).
Risk factors for in-hospital mortality in geriatric acute kidney injury patients in the ICU
A logistic regression analysis was subsequently performed to identify the independent predictors for in-hospital mortality. In the univariate analysis, hypertension, CKD, RIFLE classification, number of failing organs, need for a ventilator, need for a vasopressor, systolic blood pressure, hemoglobin level, and serum albumin level were identified as prognostic factors for in-hospital mortality (Table 4). Multivariate analyses were derived from the risk factors detected in the univariate analysis at P < 0.05 (Table 5). Hypertension (odds ratio [OR], 0.47; 95 % confidence interval [CI], 0.27–0.84; P = 0.01), CKD (OR, 2.90; 95 % CI, 1.41–5.93; P = 0.004), number of failing organs (OR, 1.65; 95 % CI, 1.23–2.22; P = 0.001), and serum albumin level (OR, 0.58; 95 % CI, 0.36–0.91; P = 0.018) were found to be independent risk factors of in-hospital mortality (Table 5). The RIFLE classification was not significantly associated with in-hospital mortality (for RIFLE-F vs. RIFLE-R: OR, 1.71; 95 % CI, 0.77–3.77; P = 0.186; for RIFLE-I vs. RIFLE-R: OR, 1.49; 95 % CI, 0.66–3.38; P = 0.336).
Discussion
AKI has emerged as a major public health problem that affects millions of patients worldwide and leads to increased mortality and progression of underlying CKD. There are few clinical studies assessing the exact incidence of AKI in the ICU. Furthermore, prior to the adoption of the RIFLE classification, these studies were often complicated by the use of differing criteria for defining AKI. Recently, using the RIFLE criteria, investigators from around the world have shown that AKI is common and results in a substantial increase in in-hospital mortality. For example, one study in northern Scotland, with a geographical population base of 523,390, found the incidence of AKI by the RIFLE classification to be 2147 cases per million population. In this study, the RIFLE classification was useful for predicting the requirement for renal replacement therapy (P < 0.001), length of hospital stay for survivors (P < 0.001), and in-hospital mortality (P = 0.035) [7]. Bagshaw et al. studied the occurrence of AKI in the ICU in 120,123 patients admitted to one of 57 ICUs across Australia [8]. The incidence of AKI stratified by the RIFLE classification was 36.1 %, with a category R in 16.2 %, I in 13.6 %, and F in 6.3 % of the patients. Each RIFLE category was independently associated with in-hospital mortality after adjusting for multiple covariates (OR: R, 2.24; I, 3.95; and F, 5.13).
Aging kidneys undergo structural and functional changes. Structural changes include vascular sclerosis, decreased weight, and increased percentages of sclerosing glomeruli [11–13]. Functional changes include decreased ultrafiltration coefficient with increased glomerular capillary pressure and declining glomerular filtration rate (GFR) [14, 15]. Thus, the structural and functional changes associated with aging alter renal sensitivity to vasodilators and vasoconstrictors, decreasing auto-regulatory capacity and reducing functional reserve. In addition, the basal rate of cellular apoptosis increases and the burst cellular proliferation in response to acute damage declines with age [16]. In response to injury, growth factors play a critical role in regulating cellular proliferation, migration, and apoptosis. The expression of these growth factors and their respective receptor transduction pathways are often downregulated with aging [17]. Reactive oxygen species, advanced glycation end products (AGEs), and levels of the anti-inflammatory AGE receptor AGER1 also increase with aging [18, 19]. These renal changes associated with aging increase risk for AKI in geriatric patients.
With the aging of the population, the incidence of geriatric patients with AKI has been increasing, and accordingly increased attention has been focused on geriatric AKI [20]. In our study, the overall in-hospital mortality of geriatric patients with AKI in the ICU was 39.8 %, and by the RIFLE classification, those in the RIFLE-R, RIFLE-I, and RIFLE-F groups were 26.4, 43.1, and 43.6 %, respectively. In previous studies, the in-hospital mortality of geriatric AKI patients in the ICU showed conflicting results because of a heterogeneous definition of the elderly, differing criteria for defining AKI, different ICU settings, different severity indices, and voluntary therapy withdrawal. Only one study has applied the RIFLE classification system to postoperative geriatric AKI patients ≥65 years in the ICU. In this study, the in-hospital mortality rates for the RIFLE-R, RIFLE-I, RIFLE-F, and overall groups were 8.5, 14.1, 35.9, and 19.9 %, respectively [9]. The higher mortality rate observed in our study may be attributed to the fact that all of our patients had been admitted to the ICU with medical problems. In our study, RIFLE classification was not significantly associated with in-hospital mortality after adjusting for multiple covariates. In fact, only a few studies have investigated the role of RIFLE classification as a prognostic factor for geriatric AKI patients in the ICU base. Chao et al. showed that in patients ≥65 years, the RIFLE classification was significantly associated with increased in-hospital mortality; however, there was no significant difference in in-hospital mortality in a subgroup >76 years of age between patients with RIFLE-R and RIFLE-I (RIFLE-I vs. RIFLE-R: OR, 1.32; P = 0.172), although the RIFLE-F patients had significantly higher mortality than both of the other groups. Another currently available risk-predicting scheme in the field of AKI, the Acute Kidney Injury Network (AKIN) classification, demonstrated poor applicability in the prediction of in-hospital mortality in geriatric AKI patients [21]. Studies have shown significantly higher in-hospital mortality in the AKIN 3 group compared with the non-AKI group; however, the AKIN 1 and 2 groups have no significant differences compared with the non-AKI group. These studies suggest that less severe categorizations of AKI by RIFLE or AKIN classification may not truly reflect the adverse impact on geriatric patients.
The application of the RIFLE classification in geriatric AKI is not expected to perform well as in the general AKI patient. A possible contributing factor may be that the RIFLE classification is based on changes in serum creatinine level or GFR, which is typically estimated based on creatinine clearance. Serum creatinine is influenced by non-renal factors such as muscle mass, nutrition, and medications. All of these can be affected by age. Therefore, RIFLE classification based on serum creatinine level does not accurately reflect the GFR in geriatric patients. In addition, geriatric AKI patient are more vulnerable to fluid accumulation, of which the dilutional effect further masks increases in serum creatinine [22]. In our study, 23 % of the patients had preexisting CKD. However, RIFLE classification is diagnosed by the frame of baseline serum creatinine, thus a higher prevalence of preexisting CKD in geriatric patients may misclassify the RIFLE category. Therefore, the nonrenal factors of serum creatinine level, greater overhydration, and inaccurate baseline creatinine denotes the erroneous assignment of the RIFLE classification in geriatric patients. Misdiagnosis, especially in milder classifications of AKI, will inflict further damage and delay appropriate nephroprotective measures such as avoidance of nephrotoxic agents, prevention of contrast-induced nephropathy, optimization of vital signs, and appropriate fluid therapy. As a result, an incorrect RIFLE classification leading to higher rates of mortality in milder classifications of AKI may not reflect the true adverse impact on geriatric patients.
We also showed that several other independent factors were predictive of increased rates of in-hospital mortality, such as hypertension, CKD, number of failing organs, and serum albumin level. These findings are comparable to previously published results. Hypertension is a significant risk factor of in-hospital mortality in geriatric patients with acute kidney injury. It is contrary to the general consensus that hypertension is considered to be a major risk factor of mortality in the general population. In fact, paradoxical aspects of hypertension in geriatric patients have been reported in previous studies. Hypertension was not a predictor of coronary and stroke mortality in geriatric patients [23]. Hypertension was a positive predictor of survival for cardiovascular mortality [24] and AKI [21] in geriatric patients. The biological reason for this is unknown, but it might be explained by better maintenance of organ perfusion in geriatric patients with an aging ischemic vasculature. It is well accepted that preexisting CKD increases a patient’s risk for developing AKI [25]. Conversely, any episode of AKI in a patient with underlying CKD inflicts additional damage on already compromised kidneys and thereby substantially increases the rate of transition to end-stage renal disease [26]. In our study, CKD was a significant risk factor of in-hospital mortality in geriatric patients with acute kidney injury, a finding that corroborates previous studies [9, 21]. However, it is unclear whether CKD patients who develop AKI have a higher burden of comorbid illness, which increases their risk for long-term mortality.
Septic shock is known to be the main cause of multiorgan failure (MOF), and the intensity of MOF is directly associated with mortality [27]. We showed that the number of failing organs was a significant risk factor of in-hospital mortality in geriatric AKI patients. However, we cannot definitively state whether MOF is a factor that represents underlying disease severity or the severity of AKI itself. Regardless of sepsis, AKI is associated with distant organ injury. It has also been associated with increased pulmonary vascular permeability and vascular congestion in animal models [28, 29]. After AKI, rapid periportal hepatocyte necrosis, vacuolization, neutrophil infiltration, and proinflammatory mRNA upregulation were also observed [30]. Small intestine histology after AKI showed profound villous lacteal capillary endothelial apoptosis, disruption of vascular permeability and epithelial necrosis. The short-term effect of ischemic AKI on inflammatory and functional changes of the brain has been studied in mice [31]. One study showed that compared with sham mice, mice with AKI had increased neuronal pyknosis and microgliosis, increased levels of the proinflammatory chemokines keratinocyte-derived chemoattractant and G-CSF in the cerebral cortex and hippocampus, and increased expression of glial fibrillary acidic protein in astrocytes in the cortex and corpus callosum. Another study demonstrated that the cellular junctions and electrical conductivity of endothelial cells from the blood–brain barrier was disrupted in mice with AKI. Acute kidney injury also leads to bone marrow and heart dysfunction [32]. MOF associated AKI might be a deleterious interorgan crosstalk arising from an imbalance of immune, inflammatory, and soluble mediator metabolism that attends severe insults to the kidney.
Serum albumin was a predictive marker of in-hospital mortality in our study. Previous studies suggest that a higher CRP-to-prealbumin ratio, a marker of nutritional status, was associated with mortality in AKI patients, independent of illness severity [33]. A meta-analysis by Wiedermann et al. provides evidence that hypoalbuminemia is a significant independent predictor of death following AKI development [34]. These data suggested that malnutrition had a negative impact on the prognosis of AKI patients. Age was not a significant risk factor in our geriatric patients. There is controversy that age per se is an important determinant of survival in geriatric patients with AKI or not. Druml et al. showed that the overall mortality did not increase with age within the group of elderly patients ≥65 years with AKI [35]. A recent retrospective cohort study of AKI in elderly adults with critical illness showed that age was an independent risk factor for AKI in elderly patients ≥55 years, although this did not seem to be a risk factor in advanced aged adults ≥75 years [36]. A large prospective study is needed to determine whether age is a risk factor of geriatric AKI patients or not. In light of our findings, geriatric AKI patients in the ICU should be assessed for severity of disease based on comorbidity, number of MOF, and nutritional status, rather than the RIFLE classification system.
Our study had certain limitations. First, as a retrospective study, the diagnosis of AKI was exclusive. The baseline serum creatinine level was unknown in 23 patients and thus was estimated using the MDRD formula with an assumed GFR for all patients of 75 mL/min per 1.73 m2. As a tool for post hoc analysis in research studies, this back-calculation method has become widespread [9, 21, 37–39]. However, previous study reported that this method led to overestimation of AKI in general ICU population, this may be our major limitation. Second, our study was conducted in a single center and only included 256 patients; therefore, the relatively small sample size may affect the analysis. Third, the definition of AKI in our study was based on serum creatinine levels. We did not assess the urine output criteria for the RIFLE classification. If the urine output criteria was accessed, the results of study might be different overcoming the limitation of serum creatinine criteria. However, urine output can be modified by additional factors independent of changes in kidney function (i.e. diuretic therapy). So urine output criteria have to be prospectively evaluated in the future study. Nonetheless, this is the first study to demonstrate the role of the RIFLE classification in geriatric AKI patients complicated by medical problems in the ICU.
In conclusion, the RIFLE classification might not be associated with mortality rates in geriatric AKI patients in the ICU. In geriatric patients, various factors besides serum creatinine levels such as comorbidity, number of MOF, and nutritional status should be considered in predicting mortality.
References
Chawla LS. Acute kidney injury leading to chronic kidney disease and long-term outcomes of acute kidney injury: the best opportunity to mitigate acute kidney injury. Contrib Nephrol. 2011;174:182–90.
Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22:28–38.
Baraldi A, Ballestri M, Rapanà R, Lucchi L, Borella P, Leonelli M, et al. Acute renal failure of medical type in an elderly population. Nephrol Dial Transplant. 1998;13:25–9.
Coca SG. Acute kidney injury in elderly persons. Am J Kidney Dis. 2010;56:122–31.
Kellum JA, Levin N, Bouman C, Lameire N. Developing a consensus classification system for acute renal failure. Curr Opin Crit Care. 2002;8:509–14.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12.
Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–8.
Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–74.
Chao CT, Wu VC, Lai CF, Shiao CC, Huang TM, Wu PC, et al. Advanced age affects the outcome-predictive power of RIFLE classification in geriatric patients with acute kidney injury. Kidney Int. 2012;82:920–7.
Lee YH, Jang MK, Kim NH, Koo JR, Kim HK, Kwon YJ, et al. Analysis of risk factors and prediction of mortality in acute renal failure treated by dialysis. Kidney Res Clin Pract. 1995;14:56–67.
Ljungqvist A, Lagergren C. Normal intrarenal arterial pattern in adult and ageing human kidney: a microangiographical and histological study. J Anat. 1962;96:285–300.
Tauchi H, Tsuboi K, Okutomi J. Age changes in the human kidney of the different races. Gerontologia. 1971;17:87–97.
Takazakura E, Sawabu N, Handa A, Takada A, Shinoda A, Takeuchi J. Intrarenal vascular changes with age and disease. Kidney Int. 1972;2:224–30.
Hoang K, Tan JC, Derby G, Blouch KL, Masek M, Ma I, et al. Determinants of glomerular hypofiltration in aging humans. Kidney Int. 2003;64:1417–24.
Anderson S, Rennke HG, Zatz R. Glomerular adaptations with normal aging and with long-term converting enzyme inhibition in rats. Am J Physiol. 1994;267:F35–43.
Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol. 2008;294:F1265–72.
Tran KT, Rusu SD, Satish L, Wells A. Aging-related attenuation of EGF receptor signaling is mediated in part by increased protein tyrosine phosphatase activity. Exp Cell Res. 2003;289:359–67.
Cai W, He JC, Zhu L, Lu C, Vlassara H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci USA. 2006;103:13801–6.
Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–91.
Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24:37–42.
Chao CT, Lin YF, Tsai HB, Wu VC, Ko WJ. Acute kidney injury network staging in geriatric postoperative acute kidney injury patients: shortcomings and improvements. J Am Coll Surg. 2013;217:240–50.
Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14:R82.
Casiglia E, Mazza A, Tikhonoff V, Pavei A, Privato G, Schenal N, et al. Weak effect of hypertensionand other classic risk factors in the elderly who have already paid their toll. J Hum Hypertens. 2002;16:21–31.
Langer RD, Ganiats TG, Barrett-Connor E. Paradoxical survival of elderly men with high blood pressure. BMJ. 1989;298:1356–7.
Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–7.
Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–8.
Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12:R158.
Kramer AA, Postler G, Salhab KF. MendezC, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55:2362–7.
Hoke TS, Douglas IS, Klein CL, He Z, FangW Thurman JM, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–64.
Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D’Agati VD, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest. 2011;91:63–84.
Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70.
Scheel PJ, Liu M, Rabb H. Uremic lung: new insights into a forgotten condition. Kidney Int. 2008;74:849–51.
Xie Q, Zhou Y, Xu Z, Yang Y, Kuang D, You H, et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol. 2011;12:30.
Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010;36:1657–65.
Druml W, Lax F, Grimm G, Schneeweiss B, Lenz K, Laggner AK. Acute renal failure in the elderly 1975-1990. Clin Nephrol. 1994;41:342–9.
Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis. 2015;65:860–9.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70.
Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–10.
Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intens Care Med. 2009;35:1692–702.
Acknowledgments
This work was supported by a 2014 clinical research grant from Pusan National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to disclose regarding this paper.
About this article
Cite this article
Shin, M.J., Rhee, H., Kim, I.Y. et al. RIFLE classification in geriatric patients with acute kidney injury in the intensive care unit. Clin Exp Nephrol 20, 402–410 (2016). https://doi.org/10.1007/s10157-015-1165-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1165-4