Abstract
Background
Fibroblast growth factor 23 (FGF23) is an important counterregulatory hormone for phosphate homeostasis. Since it has been reported that iron administration induces hypophosphatemic osteomalacia by triggering FGF23 synthesis, we hypothesized that iron administration might lead to a further increase in FGF23, resulting in alterations to Ca–P metabolism in a stage 5 CKD population.
Methods
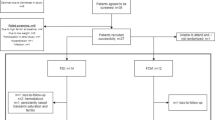
This cross-sectional study was performed in a single center, and involved 73 hemodialysis patients (47.7 ± 15.74 years old, 68.5 % men), 29 peritoneal dialysis patients (44.55 ± 15.05 years old, 62.1 % men), and 55 healthy (43.57 ± 14.36 years old, 55.6 % men) subjects. The dialysis group was subcategorized according to iron therapy administration into users and nonusers.
Results
The median iFGF23 level was significantly higher in the dialysis population than in the healthy controls [88.050 (25.2–1038.3) pg/ml versus 46.95 (2.4–356) pg/ml (p < 0.001)]. In the dialysis population, a significantly lower median iFGF23 level was observed in iron therapy users than in nonusers [87.6 (25.2–1038.3) versus 119 (51.6–1031); respectively, p = 0.045]. A significant negative association between iron administration and iFGF23 level was revealed by both univariate (r = −0.237, p = 0.016) and multivariate (β = −0.221, p = 0.032) analysis. No association was found between iFGF23 and serum ferritin and iron levels. Also, there was no association between iron therapy and serum phosphate level.
Conclusion
In contrast to what is seen for the general population, this study showed that there was a negative relationship between iron administration and serum iFGF23 level in a dialysis population. We can therefore conclude that if high levels of FGF23 are harmful, iron therapy may have a beneficial effect on bone metabolism by reducing FGF23 levels in a dialysis population.



Similar content being viewed by others
References
Saito T, Fukumoto S. Fibroblast growth factor 23 (FGF23) and disorders of phosphate metabolism. Int J Pediatr Endocrinol. 2009;2009:496514.
Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150:4835–45.
Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–31.
Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1–10.
Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–8.
Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92.
Wolf M. Fibroblast growth factor 23 and the future of phosphorus management. Curr Opin Nephrol Hypertens. 2009;18:463–8.
Filler G, Liu D, Huang SH, Casier S, Chau LA, Madrenas J. Impaired GFR is the most important determinant for FGF-23 increase in chronic kidney disease. Clin Biochem. 2011;44:435–7.
Isakova T, Wolf MS. FGF23 or PTH: which comes first in CKD ? Kidney Int. 2010;78:947–9.
Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–80.
Sato K, Nohtomi K, Demura H, Takeuchi A, Kobayashi T, Kazama J, Ozawa H. Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone. 1997;21:57–64.
Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–7.
Schouten BJ, Doogue MP, Soule SG, Hunt PJ. Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem. 2009;46:167–9.
Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, Fukumoto S, Sugimoto T, Fujita T. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–6.
Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85.
Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–8.
Okada M, Imamura K, Iida M, Fuchigami T, Omae T. Hypophosphatemia induced by intravenous administration of saccharated iron oxide. Klin Wochenschr. 1983;61:99–102.
Sato K, Shiraki M. Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr J. 1998;45:431–9.
Takeda Y, Komaba H, Goto S, Fujii H, Umezu M, Hasegawa H, Fujimori A, Nishioka M, Nishi S, Fukagawa M. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am J Nephrol. 2011;33:421–6.
Yamasaki K, Hagiwara H. Excess iron inhibits osteoblast metabolism. Toxicol Lett. 2009;191:211–5.
Seiler S, Heine GH, Fliser D. Clinical relevance of FGF-23 in chronic kidney disease. Kidney Int Suppl 2009;114:S34–S42.
Acknowledgments
This study was conducted through funding from the Gazi University Faculty of Medicine and the Postgraduate Medicine Education Association. All authors contributed to the design of the study, data collection, data interpretation, and the writing of the manuscript. Data from this manuscript were presented in part at the 2011 World Congress of Nephrology in Vancouver, Canada.
Conflict of interest
None have financial or other contractual agreements that might cause conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Deger, S.M., Erten, Y., Pasaoglu, O.T. et al. The effects of iron on FGF23-mediated Ca–P metabolism in CKD patients. Clin Exp Nephrol 17, 416–423 (2013). https://doi.org/10.1007/s10157-012-0725-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-012-0725-0