Abstract
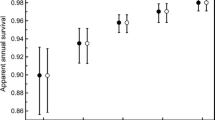
In long-lived species, juvenile survival typically is lower and more variable than adult survival, and modeling such variation is important for understanding population dynamics. Variability in juvenile survival can be related to birth- or current-year influences, and the birth-year influences can be transient, persistent, or intermediate in duration. We used multi-state models and data collected from 5,459 known-aged prebreeder female Weddell seals (Leptonychotes weddellii Lesson) tagged in Erebus Bay, Antarctica from 1980–2007 to evaluate the duration of potential birth-year influences on survival rates and the importance of birth- and current-year influences on survival and recruitment rates. Survival rates differed for each birth cohort and were positively related to current-year winter sea-ice conditions. The estimated duration of birth-cohort effects on survival was intermediate (6 years) rather than transient (2 years) or permanent. Estimated survivorship from birth to 6 years of age varied among cohorts from 0.13 (SE = 0.04) to 0.42 (SE = 0.06), and averaged 0.25 (SE = 0.02). Recruitment rates (probability of transitioning from prebreeder to breeder state) varied annually but apparently were not related to birth-year conditions. Our results provide evidence that birth- and current-year conditions act in combination to influence survival. Although for many long-lived species the influences of either birth- or current-year conditions on survival are well-studied, we suggest that modeling survival rates as a function of birth- and current-year influences simultaneously could lead to better understanding of survival and improved stochastic models to project population dynamics.





Similar content being viewed by others
References
Ainley DG, Siniff DB (2009) The importance of Antarctic toothfish as prey of Weddell seals in the Ross Sea. Antarct Sci 21:317–327
Ainley DG, Ballard G, Dugger KM (2006) Competition among penguins and cetaceans reveals trophic cascades in the western Ross Sea, Antarctica. Ecology 87:2080–2093
Arrigo KR, Thomas DN (2004) Large scale importance of sea ice biology in the Southern Ocean. Antarct Sci 16:471–486
Barbraud C, Weimerskirch H, Guinet C, Jouventin P (2000) Effect of sea-ice extent on adult survival of an Antarctic top predator: the snow petrel Pagodroma nivea. Oecologia 125:483–488
Beauplet G, Barbraud C, Chambellant M, Guinet C (2005) Interannual variation in the post-weaning and juvenile survival of subantarctic fur seals: influence of pup sex, growth rate and oceanographic conditions. J Anim Ecol 74:1160–1172
Brownie C, Hines JE, Nichols JD, Pollock KH, Hestbeck JB (1993) Capture-recapture studies for multiple strata including non-Markovian transitions. Biometrics 49:1173–1187
Burnham KP, Anderson DR (2002) Model selection and multimodel inference; a practical information-theoretic approach, 2nd edn. Springer, New York
Burns JM, Trumble SJ, Castellini MA, Testa JW (1998) The diet of Weddell seals in McMurdo Sound, Antarctica as determined from scat collections and stable isotope analysis. Polar Biol 19:272–282
Burns JM, Castellini MA, Testa JW (1999) Movements and diving behavior of weaned Weddell seal (Leptonychotes weddellii) pups. Polar Biol 21:23–36
Cam E, Link WA, Cooch EG, Monnat J, Danchin E (2002) Individual covariation in life-history traits: seeing the trees despite the forest. Am Nat 159:96–105
Cam E, Monnat J-Y, Hines JE (2003) Long-term fitness consequences of early conditions in the kittiwake. J Anim Ecol 72:411–424
Cameron MF, Siniff DB (2004) Age-specific survival, abundance, and immigration rates of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound, Antarctica. Can J Zool 82:601–615
Choquet R, Gimenez O (2012) Towards built-in capture–recapture mixed models in program E-SURGE. J Ornithol 152:625–639
Doherty PF, White GC, Burnham KP (2010) Comparison of model building and selection strategies. J Ornithol 152:317–323
Drummond H, Rodríguez C, Oro D (2011) Natural “poor start” does not increase mortality over the lifetime. Proc R Soc B 278:3421–3427
Forcada J, Trathan PN, Murphy EJ (2008) Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Global Change Biol 14:2473–2488
Gaillard J-M, Boutin J-M, Delorme D, van Laere G, Duncan P, Lebreton J-D (1997) Early survival in roe deer: causes and consequences of cohort variation in two contrasted populations. Oecologia 112:502–513
Gaillard J-M, Festa-Bianchet M, Yoccoz NG (1998) Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol Evol 13:58–63
Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst 31:367–393
Garrott RA, Rotella JJ, Siniff DB, Parkinson CL, Stauffer GE (2012) Environmental variation and cohort effects in an Antarctic predator. Oikos 121:1027–1040
Grafen A (1988) On the uses of data on lifetime reproductive success. In: Clutton-Brock TH (ed) Reproductive success: studies of individual variation in contrasting breeding systems. University of Chicago Press, Chicago, pp 454–471
Hadley GL, Rotella JJ, Garrott RA, Nichols JD (2006) Variation in probability of first reproduction of Weddell seals. J Anim Ecol 75:1058–1070
Hadley GL, Rotella JJ, Garrott RA (2007a) Influence of maternal characteristics and oceanographic conditions on survival and recruitment probabilities of Weddell seals. Oikos 116:601–613
Hadley GL, Rotella JJ, Garrott RA (2007b) Evaluation of reproductive costs for Weddell seals in Erebus Bay, Antarctica. J Anim Ecol 76:448–458
Hadley GL, Rotella JJ, Garrott RA (2008) Spatial variation in age-specific probabilities of first reproduction for Weddell seals. Oikos 117:1165–1174
Hall AJ, McConnell BJ, Barker RJ (2001) Factors affecting first-year survival in grey seals and their implications for life history strategy. J Anim Ecol 70:138–149
Hastings KK, Testa JW (1998) Maternal and birth colony effects on survival of Weddell seal offspring from McMurdo Sound, Antarctica. J Anim Ecol 67:722–740
Jenouvrier S, Barbraud C, Weimerskirch H (2006) Sea ice affects the population dynamics of Adelie penguins in Terre Adelie. Polar Biol 29:413–423
Kwok R, Comiso JC (2002) Southern Ocean climate and sea ice anomalies associated with the Southern Oscillation. J Climate 15:487–501
Laake JL (2010) RMark: R code for MARK analysis. R package version 1(9):6
Langtimm CA (2009) Non-random temporary emigration and the robust design: conditions for bias at the end of a time series. Environ Ecol Stat 3:745–761
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Madsen T, Shine R (2000) Silver spoons and snake body sizes: prey availability early in life influences long-term growth rates of free-ranging pythons. J Anim Ecol 69:952–958
Martin JGA, Festa-Bianchet M (2010) Bighorn ewes transfer the costs of reproduction to their lambs. Am Nat 176:414–423
McMahon CR, Burton HR (2005) Climate change and seal survival: evidence for environmentally mediated changes in elephant seal, Mirounga leonina, pup survival. Proc R Soc B 272:923–928
McMahon CR, Burton HR, Bester MN (2000) Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island. Antarct Sci 12:149–153
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Monaghan P (2008) Early growth conditions, phenotypic development and environmental change. Philos Trans R Soc B Biol Sci 363:1635–1645
Nichols JD, Kendall WL (1995) The use of multi-state capture-recapture models to address questions in evolutionary ecology. J Appl Stat 22:835
Pfister CA (1998) Patterns of variance in stage-structured populations: evolutionary predictions and ecological implications. Proc Natl Acad Sci USA 95:213–218
Powell LA (2007) Approximating variance of demographic parameters using the delta method: a reference for avian biologists. Condor 109:949–954
Proffitt KM, Garrott RA, Rotella JJ, Wheatley KE (2007a) Environmental and senescent related variations in Weddell seal body mass: implications for age-specific reproductive performance. Oikos 116:1683–1690
Proffitt KM, Garrott RA, Rotella JJ, Siniff DB, Testa JW (2007b) Exploring linkages between abiotic oceanographic processes and a top-trophic predator in an Antarctic ecosystem. Ecosystems 10:120–127
Proffitt KM, Garrott RA, Rotella JJ (2008a) Long-term evaluation of body mass at weaning and postweaning survival rates of Weddell seals in Erebus Bay, Antarctica. Mar Mammal Sci 24:677–689
Proffitt KM, Garrott RA, Rotella JJ (2008b) Variation in offspring sex ratio among individual Weddell seal females of different quality. Behav Ecol Sociobiol 62:1679–1687
Proffitt KM, Rotella JJ, Garrott RA (2010) Effects of pup age, maternal age, and birth date on pre-weaning survival rates of Weddell seals in Erebus Bay, Antarctica. Oikos 119:1255–1264
R Development Core Team (2012) R: a language and environment for statistical computing. R foundation for statistical computing. Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P (2003) Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J Anim Ecol 72:36–46
Rödel HG, von Holst D (2009) Features of the early juvenile development predict competitive performance in male European rabbits. Physiol Behav 97:495–502
Rotella JJ, Link WA, Nichols JD, Hadley GL, Garrott RA, Proffitt KM (2009) An evaluation of density-dependent and density-independent influences on population growth rates in Weddell seals. Ecology 90:975–984
Smith MSR (1965) Seasonal movements of the Weddell seal in McMurdo Sound, Antarctica. J Wildlife Manage 29:464–470
Stenseth NC, Ottersen G, Hurrell JW, Mysterud A, Lima M, Chan K, Yoccoz NG, Ådlandsvik B (2003) Review article. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc R Soc B 270:2087–2096
Testa JW, Siniff DB, Ross MJ, Winter JD (1985) Weddell seal—Antarctic cod interactions in McMurdo Sound. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Proceedings of the 4th SCAR symposium on Antarctic biology. Springer, New York, pp 561–565
Testa JW, Oehlert G, Ainley DG, Bengtson JL, Siniff DB, Laws RM, Rounsevell D (1991) Temporal variability in Antarctic marine ecosystems—periodic fluctuations in the Phocid seals. Can J Fish Aquat Sci 48:631–639
van de Pol M, Bruinzeel LW, Heg D, van der Jeugd HP, Verhulst S (2006) A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus). J Anim Ecol 75:616–626
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl):120–138
Wilson PR, Ainley DG, Nur N, Jacobs SS, Barton KJ, Ballard G, Comiso JC (2001) Adélie penguin population change in the pacific sector of Antarctica: relation to sea-ice extent and the Antarctic Circumpolar Current. Mar Ecol Prog Ser 213:301–309
Yuan X (2004) ENSO-related impacts on Antarctic sea ice: a synthesis of phenomenon and mechanisms. Antarct Sci 16:415–425
Acknowledgments
This work was supported by the National Science Foundation, Office of Polar Programs (Grant No. ANT-0635739 to R. A. Garrott, J. J. Rotella, and D. B. Siniff, and previous grants to D. B. Siniff and J. W. Testa). Animal handling protocol was approved by Montana State University’s Institutional Animal Care and Use Committee (Protocol #41-05). Raytheon Polar Services Corporation, Petroleum Helicopters International, and the New York National Guard provided logistical support for field work in Antarctica. W. L. Kendall, T. E. McMahon, and D. B. Siniff and 2 anonymous reviewers provided useful comments on previous manuscript drafts. Many field assistants assisted with data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stauffer, G.E., Rotella, J.J. & Garrott, R.A. Birth-year and current-year influences on survival and recruitment rates of female Weddell seals. Popul Ecol 55, 405–415 (2013). https://doi.org/10.1007/s10144-013-0379-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-013-0379-0