Abstract
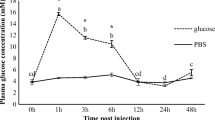
The present work was designed to assess the effects of artificially increased high plasma cortisol levels induced by slow-release cortisol implants on the mRNA abundance of the glucocorticoid receptor (GR) in different organs of Sparus aurata (Gilthead sea bream), as well as to evaluate global transcriptional changes in the liver, using the Aquagenomics S. aurata oligo-nucleotide microarray technology. For that purpose, groups of fish were intraperitoneally injected with implants containing two different concentrations of cortisol (50 or 200 μg/g body weight). Blood and organs were sampled after 7 and 14 days of cortisol implantation. Only fish with 200 μg/g implants exhibited a significant rise in plasma cortisol. Thus, we evaluated the expression of the GR in different organs in these fish 7 and 14 days post-implantation. GR mRNA abundance was upregulated in head kidney and heart of fish at both sampling times. In liver and muscle, GR mRNA abundance was upregulated after 14 days, whereas in gills, the GR mRNA transcript was upregulated earlier, at day 7. These results suggest that increased plasma cortisol induced by a slow-release implant of cortisol mimics the overall effects of stress and affects the expression of GR mRNA in a time- and organ-specific manner. Data obtained with the Aquagenomics S. aurata oligo-nucleotide microarray allowed the identification of a total of 491 cortisol-responsive transcripts and highlight the strong intensity of transcriptional modulation in liver of fish implanted with cortisol after 7 days, in contrast to that observed at day 14. Transcriptional remodeling highlighted a significant activity in carbohydrate metabolism mainly in the gluconeogenic pathway linked to downregulation of inflammatory and immune response processes in implanted fish.




Similar content being viewed by others
References
Acerete L, Balasch JC, Castellana B, Redruello B, Roher N, Canario AV, Planas JV, MacKenzie S, Tort L (2007) Cloning of the glucocorticoid receptor (GR) in gilthead sea bream (Sparus aurata) differential expression of GR and immune genes in gilthead sea bream after an immune challenge. Comp Biochem Physiol B 148:32–43
Adem S, Ciftci M (2012) Purification of rat kidney glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and glutathione reductase enzymes using 2′,5′-ADP Sepharose 4B affinity in a single chromatography step. Protein Expres Purif 81:1–4
Alsop D, Vijayan MM (2009) The zebrafish stress axis. Molecular fallout from the telesot specific genome duplication event. Gen Comp Endocrinol 16:62–66
Aluru N, Vijayan MM (2007) Hepatic transcriptome response to glucocorticoid receptor activation in rainbow trout. Physiol Genom 31:483–491
Aluru N, Vijayan MM (2009) Stress transcriptomics in fish: a role for genomic cortisol signaling. Gen Comp Endocrinol 164:142–150
Bourdineaud JP, Baudrimont M, Gonzalez P, Moreau JL (2006) Challenging the model for induction of metallothionein gene expression. Biochimie 88:1787–1792
Bury NR, Sturm A, Le Rouzic P, Lethimonier C, Ducouret B, Guiguen Y, Robinson-Rechavi M, Laudet V, Rafestin-Oblin ME, Prunet P (2003) Evidence for two distinct functional glucocorticoid receptors in teleost fish. J Mol Endocrinol 31:141–156
Calduch-Giner JA, Davey G, Saera-Vila A, Houeix B, Talbot A, Prunet P, Cairns MT, Pérez-Sánchez J (2010) Use of microarray technology to assess the time course of liver stress response after confinement exposure in gilthead sea bream (Sparus aurata L.). BMC Genom 11:193, http://www.biomedcentral.com/1471-2164/11/193
Dargemont C, Ossareh-Nazari B (2012) Cdc48/p97, a key actor in the interplay between autophagy and ubiquitin/proteasome catabolic pathways. Biochim Biophys Acta 1823:138–144
Golicnik M, Olguin LF, Feng G, Baxter NJ, Waltho JP, Williams NH, Hollfelder F (2009) Kinetic analysis of beta-phosphoglucomutase and its inhibition by magnesium fluoride. J Am Chem Soc 131:1575–1588
Holland MCH, Lambris J (2002) The complement system in teleosts. Fish Shellfish Immunol 12:399–420
Kayes-Wandover KM, White PC (2000) Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab 85:2519–2525
Kim JW, Dang CV (2005) Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30:142–150
Laiz-Carrión R, Rio MPM, Miguez JM, Mancera JM, Soengas JL (2003) Influence of cortisol on osmoregulation and energy metabolism in gilthead sea bream Sparus aurata. J Exp Zool 298A:105–118
Liang L, Zhao M, Xu Z, Yokoyama KK, Li T (2003) Molecular cloning and characterization of CIDE-3, a novel member of the cell-death-inducing DNA-fragmentation-factor (DFF45)-like effector family. Biochem J 370:195–203
MacKenzie S, Iliev D, Liarte C, Koskinen H, Planas JV, Goetz FW, Mölsä H, Krasnov A, Tort L (2006) Transcriptional analysis of LPS-stimulated activation of trout (Oncorhynchus mykiss) monocyte/macrophage cells in primary culture treated with cortisol. Mol Immunol 43:1340–1348
Martínez-Porchas M, Martínez-Córdova LR, Ramos-Enriquez R (2009) Cortisol and glucose: reliable indicators of fish stress? Pan-Am J Aquat Sci 4:158–178
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fisheries 9:211–268
Momoda TS, Schwindt AR, Feist GW, Gerwick L, Bayne CJ, Schreck CB (2007) Gene expression in the liver of rainbow trout, Oncorhynchus mykiss, during the stress response. Comp Biochem Physiol D 2:303–315
Montero D, Tort L, Izquierdo MS, Robaina L, Vergara JM (1998) Depletion of serum alternative complement pathway activity in gilthead seabream caused by α-tocopherol and n-3 HUFA dietary deficiencies. Fish Physiol Biochem 18:399–407
Ortuño J, Esteban A, Meseguer J (2002) Lack of effect of combining different stressors on innate immune responses of sea bream (Sparus aurata L.). Vet Immunol Immunopathol 84:17–27
Ribas L, Planas JV, Barton B, Monetti C, Bernadini G, Saroglia M, Tort L, MacKenzie S (2004) A differentially expressed enolase gene isolated from the gilthead sea bream (Sparus aurata) under high-density conditions is up-regulated in brain after in vivo lipopolysaccharide challenge. Aquaculture 241:195–206
Roncarati A, Melotti P, Dees A, Mordenti O, Angellotti L (2006) Welfare status of cultured seabass (Dicentrarchus labrax L.) and seabream (Sparus aurata L.) assessed by blood parameters and tissue characteristics. J Appl Ichthyol 22:225–234
Saera-Vila A, Calduch-Giner JA, Gómez-Requeni P, Médale F, Kaushik S, Pérez-Sánchez J (2005) Molecular characterization of gilthead sea bream (Sparus aurata) lipoprotein lipase. Transcriptional regulation by season and nutritional condition in skeletal muscle and fat storage tissues. Comp Biochem Physiol B 142:224–232
Stolte EH, van Kemenade BMLV, Savelkoul HFJ, Flik G (2006) Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J Endocrinol 190:17–28
Stolte EH, de Mazon AF, Leon-Koosterziel KM, Jesiak M, Bury NR, Sturm A, Savelkoul HF, van Kemenade BM, Flik G (2008) Corticosteroid receptors involved in stress regulation in common carp, Cyprinus carpio. J Endocrinol 198:403–417
Sunyer JO, Tort L (1995) Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet Immunol Immunopathol 45:333–345
Tadiso TM, Krasnov A, Skugor S, Afanasyev S, Hordvik I, Nilsen F (2011) Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genomics 12:141. doi:10.1186/1471-2164-12-141
Terova G, Gornati R, Rimoldi S, Bernardini G, Saroglia M (2005) Quantification of a glucocorticoid receptor in sea bass (Dicentrarchus labrax, L.) reared at high stocking density. Gene 357:144–151
Tort L (2010) Stress in farmed fish. Its consequences in health and performance. In: Koumoundouros G (ed) Recent advances in aquaculture research. Transworld Research Network. ISBN 978-81-7895-7. Trivandrum, Kerala, India
Tort L (2011) Stress and immunomodulation in fish. Develop Comp Immunol 35:1366–1375
Tort L, Gomez E, Montero D, Sunyer JO (1996) Serum haemolytic and agglutinating activity as indicators of fish immunocompetence: their suitability in stress and dietary studies. Aquacult Int 4:31–41
Vazzana M, Vizzini A, Sanfratello MA, Celi M, Salerno G, Parrinello N (2010) Differential expression of two glucocorticoid receptors in seabass (teleost fish) head kidney after exogeneous cortisol inoculation. Comp Biochem Physiol A 157:49–54
Vijayan MM, Raptis S, Sathiyaa R (2003) Cortisol treatment affects glucocorticoid receptor and glucocorticoid-responsive genes in the liver of rainbow trout. Gen Comp Endocrinol 132:256–263
Winston GW (1991) Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C 100:173–176
Wiseman S, Osachoff H, Bassett E, Malhotra J, Bruno J, VanAggelen G, Mommsen TP, Vijayan MM (2007) Gene expression pattern in the liver during recovery from an acute stressor in rainbow trout. Comp Biochem Physiol D 2:234–244
Yada T, Azuma T, Hyodo S, Hirano T, Grau EG, Schreck CB (2007) Differential expression of corticosteroid receptor genes in rainbow trout (Oncorhynchus mykiss) immune system in response to acute stress. Can J Fish Aquat Sci 64:1382–1389
Yada T, Hyodo S, Schreck CB (2008) Effects of seawater acclimation on mRNA levels of corticosteroid receptor genes in osmoregulatory and immune systems in trout. Gen Comp Endocrinol 156:622–627
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27:647–659
Zhonghua C, Chunpin G, Yong Z, Kezhi X, Yaou Z (2008) Cloning and bioactivity analysis of a CXC ligand in black seabream Acanthopagrus schlegeli: the evolutionary clues of ELR + CXC chemokines. BMC Immunol 9:66. doi:10.1186/1471-2172-9-66
Zilli L, Beirão J, Schiavone R, Herraez MP, Cabrita E, Storelli C, Vilella S (2011) Aquaporin inhibition changes protein phosphorylation pattern following sperm motility activation in fish. Theriogenology 76:737–744
Acknowledgments
This work was supported by the Plan Nacional de Investigación (BFU2009-07354, and Consolider-Aquagenomics (CSD 2007-00002), Government of Spain. Financial support to Mariana Teles provided by FCT (SFRH/BPD/34449/2006, Government of Portugal) and (JCI-2010-06509 Government of Spain) is thankfully acknowledged. Thanks are also given to Roger Traveset, Pilar Tudela, and Neus Riuet for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teles, M., Boltaña, S., Reyes-López, F. et al. Effects of Chronic Cortisol Administration on Global Expression of GR and the Liver Transcriptome in Sparus aurata . Mar Biotechnol 15, 104–114 (2013). https://doi.org/10.1007/s10126-012-9467-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-012-9467-y