Abstract
Background
Gastric “crawling-type” adenocarcinoma (CTAC) is a neoplasm histologically comprising irregularly fused glands with low-grade cellular atypia that tends to spread laterally in the mucosa. It is necessary to elucidate the clinicopathological characteristics of CTAC.
Methods
We evaluated 25 CTACs–16 intramucosal (M-) and 9 submucosal invasive (SM-) cancers–clinicopathologically and immunohistochemically.
Results
CTAC was most frequently located in the lesser curvature of the middle-third of the stomach. Macroscopically, 21 lesions were superficial-depressed and 4 were superficial-flat type. Histologically, all CTACs had cystic dilated glands and 16 lesions had focal signet-ring cells. All invasive areas of the SM-CTACs were occupied by poorly differentiated adenocarcinoma with an infiltrative growth pattern and abundant stroma. Fifteen CTACs were surrounded by mucosa with partial or no intestinal metaplasia. In the intramucosal area, 24 lesions were mixed phenotype with mucin and brush border immunoexpression. SM-CTAC was frequent in lesions with an intramucosal poorly differentiated component (PDC) greater than 10 mm in size (P = 0.041), and lymph node metastasis (LNM) was frequent in lesions with a PDC greater than 20 mm (P = 0.039). The frequency of an expanded pattern (Ki-67-positive cells occupying > 50 % of the mucosa) was higher in SM-CTAC than in M-CTAC (P = 0.027). p53 overexpression was not detected in the intramucosal areas of any of the lesions.
Conclusion
CTAC is a distinct subgroup of gastric adenocarcinoma in the early phase. A larger PDC and a Ki-67 expanded pattern were predictive of submucosal invasion or LNM.
Similar content being viewed by others
Introduction
The Laurén classification divides gastric adenocarcinomas into two main histological types: intestinal and diffuse, depending on the degree of glandular formation [1]. From a histogenetic point of view, these two types correspond roughly to differentiated- and undifferentiated-type adenocarcinomas in the Nakamura classification [2, 3]. That is, differentiated-type adenocarcinomas arise from mucosa with intestinal metaplasia and undifferentiated-type adenocarcinomas originate from mucosa without intestinal metaplasia. In the WHO classification [4] and in the Japanese classification of gastric carcinoma [5], gastric adenocarcinoma is graded as well-, moderately, and poorly differentiated, according to the histological extent of glandular formation.
Endoscopic treatment for early gastric cancer has become widespread. According to the Japanese gastric cancer treatment guidelines 2010, endoscopic resection should be considered for tumors with a very low risk of lymph node metastasis (LNM) [6, 7]. Consequently, absolute indications for endoscopic resection are restricted to clinically diagnosed intramucosal well- or moderately differentiated adenocarcinomas smaller than 2.0 cm in diameter and without ulcerative changes.
Tumors with components of both well- to moderately differentiated and poorly differentiated adenocarcinoma are occasionally found, even in the early disease phase [8, 9]. The evidence for the risk of LNM in such tumors remains insufficient, however, and it remains controversial whether endoscopic resection is appropriate for such lesions. Therefore, the clinicopathological characteristics and risk factors for LNM in such tumors should be clarified. In this study, we focused on “crawling-type” adenocarcinoma (CTAC), which is considered to be one of such tumors.
“Crawling-type” adenocarcinoma (CTAC) was described by Takizawa et al. [10, 11] as a tumor often spreading laterally (“crawling”) into the mucosal layer. Histologically, this type of carcinoma comprises irregularly fused glands with low-grade cellular atypia in the epithelial proliferative zone. Occasionally, signet-ring cells are found. Although the importance of recognizing this subgroup has been mentioned in other review articles [12, 13], the clinicopathological characteristics of CTAC have not been adequately described.
In the present study, to clarify the clinicopathological characteristics of CTAC, we examined samples from 25 cases of CTAC–16 intramucosal (M-) and 9 submucosal invasive (SM-) carcinomas. In addition, the clinical implications of a diagnosis of CTAC and predictive histological risk factors for submucosal invasion and LNM are also discussed.
Patients, materials, and methods
Patients and lesions
“Crawling-type” adenocarcinomas were collected from a total of 691 cases of pT1 primary gastric cancer in patients without any preceding treatments. Patients included in this study underwent surgical intervention at Tokyo Medical and Dental University Hospital from January 2002 to July 2011, or underwent endoscopic submucosal dissection at Kudanzaka Hospital from October 2003 to July 2011. We examined 606 surgically resected and 270 endoscopically resected specimens, including simultaneous or metachronous multiple lesions, from these patients. After formalin fixation and paraffin embedding, specimens from the surgically resected samples were cut into long strips 5 mm wide and those from endoscopically resected samples were cut into long strips 2 mm wide. Some sections (3-μm-thick) were stained with hematoxylin–eosin and elastica van Gieson to evaluate histological findings, and other sections were used for immunohistochemistry (IHC). Histological diagnosis of CTAC was made according to the criteria proposed by Takizawa et al. [10, 11]. Adenocarcinomas including at least some of the CTAC components were obtained for this study. Two independent observers (N.O. and H.K.) evaluated the histopathological findings and IHC results. In any disagreements between the two observers, the observers re-evaluated the specimens and reached a consensus after discussion.
This study was performed in accordance with the World Medical Association’s Declaration of Helsinki. The ethics committees of Tokyo Medical and Dental University and Kudanzaka Hospital approved the protocols.
Clinicopathological findings and histological evaluation of CTAC
Patients’ age, sex, therapeutic method, tumor location, macroscopic type including the presence of ulcerative changes, and LNM status were obtained from pathology records in each hospital. For M-CTACs, tumor size was evaluated as the maximum diameter, whereas for SM-CTACs the maximum diameters of both the intramucosal area and the submucosal invasive area were evaluated.
All lesions were classified as M- or SM-cancer based on the depth of invasion. Further, in SM-cancers, the vertical distances from the muscularis mucosa to the invasive front were measured. Lesions less than 500 μm away from the muscularis mucosa were subclassified as SM1, and lesions 500 μm or more away from the muscularis mucosa were subclassified as SM2, according to the Japanese classification of gastric carcinoma [5]. Lymphatic and venous permeations were graded as positive or negative in hematoxylin–eosin-stained sections and in elastica van Gieson-stained sections, respectively.
The stromal volume and tumor infiltration pattern were evaluated according to the Japanese classification of gastric carcinoma [5] as follows. The stromal volume is classified into three categories: medullary-type (med), which has scanty stroma; scirrhous-type (sci), which has abundant stroma; and intermediate-type (int), which is intermediate between med and sci. The tumor infiltration pattern was also classified into three categories: INFa, in which tumors display expanding growth with a distinct border separating them from the surrounding tissue; INFc, in which tumors display an infiltrative growth pattern with no distinct border; and INFb, in which tumors show an intermediate pattern between INFa and INFc.
Condition of the surrounding mucosa
The mucosa surrounding the lesions was examined histologically. Both proximal and distal sides were divided into fundic gland mucosa, pyloric gland mucosa, and intermediate zone mucosa. The degree of intestinal metaplasia (IM) was classified as diffuse IM, partial IM, or without IM.
IHC
Sections from one or two representative paraffin blocks of each case were prepared for immunohistochemical staining. We used monoclonal antibodies to human gastric mucin (HGM; clone 45M1, Novocastra, Newcastle upon Tyne, UK, diluted 1:50), MUC6 glycoprotein (MUC6; clone CLH5, Novocastra, diluted 1 : 50), MUC2 glycoprotein (MUC2; clone CCP58, Novocastra, diluted 1:100), CD10 protein (CD10; clone 56C6, Novocastra, diluted 1:100), Cdx2 protein (Cdx2; clone AMT28, Novocastra, diluted 1:50), p53 protein (p53; clone DO-7, Novocastra, diluted 1:1000), and Ki-67 antigen (Ki-67; clone MIB-1, Dako, Glostrup, Denmark, diluted 1:800). All sections were incubated with a primary antibody at its working dilution for 60 min at room temperature (HGM and Cdx2) or for 24 h at 4 °C (MUC6, MUC2, CD10, p53, and Ki-67), subsequent to antigen retrieval. Antigen retrieval was performed using a microwave at 97 °C for 40 min (HGM and Cdx2), or an autoclave at 121 °C for 20 min (MUC6, MUC2, CD10, p53, and Ki-67). Sections were stained to detect each antigen using a Vectastain ABC Immunoperoxidase kit (Vector Laboratories, Burlington, CA, USA) or an EnVision+ System (Dako, Glostrup, Denmark). The sections were incubated in 3,3′-diaminobenzidine for 10 min, which stained the antigen brown, and then counterstained with Mayer’s hematoxylin.
Phenotypic classification based on the presence of mucin, brush border, and Cdx2 expression
We classified lesions as having one of four phenotypes: gastric phenotype (G-phenotype), in which 10 % or more of the cancer cells were immunoreactive for HGM and/or MUC6, fewer cells were stained for MUC2 and CD10, and cancer cells were weakly positive or negative for Cdx2; intestinal phenotype (I-phenotype), in which 10 % or more of the cancer cells were immunoreactive for MUC2 and/or CD10, or strongly positive for Cdx2, and also in which fewer cells were stained for HGM and MUC6; mixed phenotype, in which 10 % of the cancer cells were immunoreactive for HGM and/or MUC6, and were also immunoreactive for at least one of MUC2, CD10, and Cdx2; and null phenotype (N-phenotype), in which fewer than 10 % of the cancer cells were immunoreactive for any of the five antigens. Further, the mixed phenotype was classified into two subgroups as follows: mixed gastric phenotype (MG-phenotype), in which cancer cells were predominantly immunoreactive for HGM and/or MUC6; and mixed intestinal phenotype (MI-phenotype), in which cancer cells were predominantly immunoreactive for MUC2 and/or CD10, and/or Cdx2. These definitions are based on the definitions of Egashira et al. [14], and according to the results of Mizoshita et al. [15, 16].
Quantitative evaluation of intramucosal poorly differentiated component (PDC)
The PDC was defined as an intramucosal component composed of signet-ring cell carcinoma and/or poorly differentiated adenocarcinoma, without tubular or papillary structures. The maximum diameter of the PDC was measured and used for quantitative evaluation.
Distribution pattern of Ki-67-positivity
The lesions were divided into two groups, as follows, based on the distribution pattern of the Ki-67-positive cancer cells in the intramucosal area: limited pattern, Ki-67-positive cancer cells showed zonal distribution and occupied 50 % or less of the mucosa; and expanded pattern, Ki-67-positive cells showed zonal distribution and occupied more than 50 % of the mucosa, or showed diffuse distribution.
p53 overexpression
The p53 overexpression was defined as positive when intensely stained cancer cells were distributed diffusely. Weak and scattered staining was considered negative. The definition was based on the definitions of Fukunaga et al. [17] and Gabbert et al. [18].
Statistical analysis
Fisher’s exact probability test, or the Wilcoxon signed-ranks test were used for analysis of the differences between groups. Differences with P values of less than 0.05 were considered to be statistically significant. StatView software (version 5.0; SAS Institute, Cary, NC, USA) was used for the statistical analyses.
Results
Clinicopathological and histological findings
Twenty-five (2.9 %) CTAC lesions were selected from the 876 examined lesions. Representative cases with endoscopic, macroscopic, and microscopic pictures are shown in Figs. 1, 2, and 3. The clinicopathological features of the 25 CTACs are listed in Table 1. Sixteen lesions were M- and 9 lesions were SM-cancer. The patients comprised 21 men and 4 women, ranging in age from 40 to 84 years (median 61 years). Nineteen (76 %) lesions were located in the middle-third of the stomach and 10 (40 %) lesions were located in the lesser curvature. The maximum diameters of the tumors ranged from 16 to 185 mm (median 33 mm). In the SM-CTACs, the maximum diameters of the lesions were significantly larger in the intramucosal area than in the submucosal invasive area (median 35 vs. 15 mm, P = 0.008). In regard to the macroscopic type, 21 (84 %) lesions were classified as superficial-depressed type (Figs. 1c–d, 3c) and the remaining 4 (16 %) lesions were classified as superficial-flat type (Fig. 2c).
Intramucosal crawling-type adenocarcinoma (case no. 1). a, b On endoscopy, shallow depressed lesion at the lesser curvature of the posterior wall and a slightly elevated lesion at the anterior wall were recognized in the lower body of the stomach (a regular endoscopic view, b endoscopic view after spraying indigocarmine). Demarcation of the lesions was indistinct. c, d Macroscopic view of a distal gastrectomy specimen (c low-power magnification, d high-power magnification). A slightly depressed lesion with ulcer scar formation was recognized. Demarcation of the tumor (indicated by yellow arrowheads) was indistinct. e–g Histological micrographs of the resected specimen. Irregularly fused glands (e, f indicated by black arrowheads in f) and cystic dilated glands (g) were found in the mucosa. Cellular atypia was not prominent (e low-power magnification, f high-power magnification of black square in e, g high-power magnification)
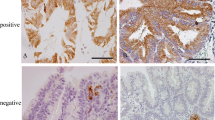
Intramucosal crawling-type adenocarcinoma (case no. 7). a, b Endoscopic view revealed a shallow depressed lesion in the anterior wall of the gastric angle. The color of the mucosa showed a mosaic pattern containing small foci of normal-like mucosa. Demarcation of the lesion was partially indistinct but recognizable (a regular endoscopic view, b endoscopic view after spraying indigocarmine). c Macroscopic view of the endoscopic submucosal dissection specimen. Demarcation of the tumor is indicated by yellow arrowheads. d Histological micrograph with low-power magnification. Cancer glands are mainly located in the epithelial proliferative zone. e–g On high-power magnification, irregularly fused glands with low-grade cellular atypia (e indicated by black arrowheads, high-power magnification of the black square in d), cystic dilated glands with slightly eosinophilic cuboidal cytoplasm (f indicated by black arrowheads), and small foci of signet-ring cells (g indicated by black arrowheads) were seen. Immunostaining pattern is shown (i–n) together with H&E staining of semi-serial histological slide (h). Cancer cells revealed positivity for HGM (i), MUC6 (j), and MUC2 (k) and weakly staining for CD10 (l); most of the cancer cells were weakly stained for Cdx2 (m). This case was classified as mixed gastric (MG)-phenotype. p53 overexpression was not detected (n). Ki-67-positive cells showed a limited pattern (o)
Submucosal invasive crawling-type adenocarcinoma (case no. 19). a, b Endoscopic view reveals a shallow depressed lesion with an ulcerative scar in the middle body of the gastric angle. The color of the mucosa formed a mosaic pattern. Demarcation of the lesion was indistinct (a regular endoscopic view, b endoscopic view after spraying indigocarmine). c Macroscopic view of the distal gastrectomy specimen. Demarcations of the mucosal area and submucosal invasive area are indicated by yellow arrowheads and red dots, respectively. d, e Histological micrographs of the surgical specimen. Irregularly fused glands with low-grade cellular atypia mimicking intestinal metaplasia (indicated black arrowheads in e) are shown (d low-power magnification, e high-power magnification of the indicated area by the open black square in d). f Histological micrograph of the area of submucosal invasion. Cancer cells had invaded the submucosal layer. Muscularis mucosa (MM) is shown (low-power magnification). g, In the intramucosal area, irregularly fused glands with poorly differentiated components (indicated by open yellow square in f) were found. h In the submucosal area, cancer cells without tubular components are shown. The distance from the MM to the invasive front was measured as 400 μm (indicated by the open red square in f)
Histologically, irregularly fused tumor glands with low-grade cellular atypia were located mainly in the epithelial proliferative zone (Figs. 1e–f, 2d–e). Almost all of the tumor glands included goblet cells and occasionally seemed to mimic IM. In all lesions, cystic dilated glands comprising cells with slightly eosinophilic and cuboidal cytoplasm were found focally among the irregularly fused glands (Figs. 1g, 2f). Further, foci of signet-ring cells (Fig. 2g) were found in 16 of the 25 (64 %) lesions. In all SM-CTACs, tumor tissues in the submucosal invasive area were composed of poorly differentiated adenocarcinoma with an infiltrative growth pattern and abundant stroma (Fig. 3h). In the SM-CTACs, 2 lesions exhibited lymphatic permeation and 1 lesion exhibited venous permeation. LNM was detected in 4 lesions–1 M-CTAC and 3 SM-CTACs. In the M-CTACs, 8 lesions (50 %), including 1 lesion with LNM, had ulcerative changes. There were no significant correlations between the frequency of LNM and depth of invasion, presence of ulcerative changes, or vessel permeation. In regard to the stromal volume and tumor infiltration pattern, sci (7 of 9, 78 %) and INFc (5 of 9, 56 %), respectively, were the most frequent groups. Lesions showing scanty stroma (med) or an expansive growth pattern (INFa) were not observed.
Condition of the surrounding mucosa
Ten (63 %) M-CTACs and 5 (56 %) SM-CTACs were surrounded by non-cancerous gastric mucosa with partial IM or without IM, whereas 6 (37 %) M- and 4 (44 %) SM-CTACs were surrounded by non-cancerous gastric mucosa with diffuse IM. The degrees of IM were not significantly different between M- and SM-CTACs. In M-CTACs, 9 (56 %) lesions were located in the intermediate zone mucosa. Furthermore, 8 (50 %) lesions were located on the boundary line between fundic gland mucosa and pyloric gland mucosa, irrespective of the presence of IM [2].
Phenotypic classification
The phenotypic classification of CTAC is summarized in Table 2. Twenty-four (96 %) lesions–all M-CTACs and 8 SM-CTACs–were of the mixed phenotype. In those lesions, the MG-phenotype (17 lesions) was more frequent than the MI-phenotype (7 lesions). Only one SM-CTAC was classified as the G-phenotype. In the submucosal invasive area, various phenotypic expressions, including the I- or N-phenotype, were found. The phenotypic classification of early gastric adenocarcinoma in the literature [9, 14, 15, 19, 20], including this study, is summarized in Table 3. The frequency of the mixed phenotype was higher in CTAC than in well- to moderately differentiated adenocarcinoma (P < 0.001) and poorly differentiated adenocarcinoma (P < 0.001). The frequency of the G-phenotype was higher in poorly differentiated adenocarcinoma than in well- to moderately differentiated adenocarcinoma (P = 0.029) and CTAC (P < 0.001). The frequency of the I-phenotype was higher in well- to moderately differentiated adenocarcinoma than in CTAC (P < 0.001) and poorly differentiated adenocarcinoma (P < 0.001).
Quantitative evaluation of PDC
An intramucosal poorly differentiated component was found in 19 lesions, 10 of 16 M-CTACs and 9 of 9 SM-CTACs. Maximum diameters of PDC in each lesion are shown in Table 1. Relationships between the maximum diameter of PDC and depth of invasion, and status of LNM are shown in Table 4. The frequency of SM-CTAC in lesions with PDC greater than 10 mm was significantly higher than the frequency in those with PDC 10 mm or smaller (53 vs. 10 %, P = 0.041). LNM was not found in lesions with PDC 20 mm or less, whereas LNM was found in 4 of 12 (33 %) lesions with PDC greater than 20 mm (P = 0.039).
Distribution pattern of Ki-67-positivity
The relationship between the Ki-67 distribution pattern and the depth of invasion and the LNM status is shown in Table 4. The frequency of the limited pattern was significantly higher in M-CTAC than in SM-CTAC (44 vs. 0 %, P = 0.027). The sensitivity and specificity of the expanded pattern for SM-CTAC were 100 and 44 %, respectively. LNM was found only in lesions with an expanded pattern, although there was no statistical significance in LNM frequency between the limited pattern and the expanded pattern (P = 0.30). The sensitivity and specificity of the expanded pattern for LNM were 100 and 33 %, respectively.
Relationships among PDC, Ki-67, depth of invasion, and LNM status
Relationships among PDC, Ki-67, depth of invasion, and LNM status are shown in Table 4. The frequency of lesions both with PDC greater than 10 mm and an expanded pattern was significantly higher in SM-CTAC than in M-CTAC (89 vs. 38 %, P = 0.033). Further, LNM was found only in lesions with both PDC greater than 20 mm and an expanded pattern, and such lesions were significantly more frequent than other lesions (P = 0.026).
p53 overexpression
p53 overexpression was not observed in the intramucosal areas of any of the lesions. Of the 9 SM-CTACs, only 1 lesion had p53 overexpression in the submucosal invasive area.
Discussion
In the present study, we elucidated the unique features of CTAC, which should be discriminated as a distinct subgroup of gastric cancer. The implications for the diagnosis of CTAC have been described previously in several Japanese reports and review articles [10–13]. To our knowledge, however, there are no systematic studies of CTAC in which surgically or endoscopically resected specimens have been used.
Macroscopically, CTACs are frequently found in the middle-third of the stomach and are located in the intermediate zone. Further, CTAC is characterized as being superficial-depressed (0–IIc) or superficial-flat (0–IIb) type. Takizawa et al. [10, 11] have mentioned that the cancer margin of CTAC is occasionally indistinct. Such a macroscopic characteristic may be due to the fact that CTAC tumor glands “crawl” into the epithelial proliferative zone where they are often at least partly covered by non-neoplastic foveolar epithelium. Ninomiya et al. [21] observed similar histological findings in some poorly differentiated adenocarcinomas (diffuse-type); thus, particular attention must be paid to determine the cancer margin. Because the cancer margin may be difficult to determine endoscopically in CTAC [10, 11], the possibility of CTAC must be considered when superficial-depressed or superficial-flat lesions are found in the middle-third of the stomach, with careful evaluation of the demarcation.
Histological analysis in the present study confirmed that irregularly fused glands were the most important diagnostic clue for CTAC. To emphasize the importance of these glands, Kato et al. [12] proposed the nickname “WHYX lesion” because of the similarity between these glands and the alphabet letters W, H, Y, and X. Because cancer cells of CTAC showed low-grade cytological atypia, tumor glands may be overlooked, especially in small biopsy specimens. Therefore, careful attention should be paid to architectural abnormalities such as irregularly fused glands on microscopic examination. In addition, cystic dilated glands and foci of signet-ring cells were considered supportive findings for a diagnosis of CTAC in this study. In benign ulcerative lesions due, for example, to transcatheter arterial embolization, however, non-neoplastic degenerative glands may mimic the cystic dilated glands of CTAC [10]. To discriminate these glands, Takizawa has commented that the degenerative glands located in deeper sites of the mucosa and the neoplastic glands of CTAC are observed in the epithelial proliferative (middle) zone [10].
Jass [22] and Filipe et al. [23] frequently observed IM surrounding gastric carcinoma, especially in well- to moderately differentiated adenocarcinoma, and suggested that IM is likely to become malignant; in other words, IM may be a precancerous lesion. On the other hand, previous histopathological and experimental analyses have shown that a causal relationship between IM and carcinoma is unlikely, and these studies have suggested that IM is important not as a precancerous lesion but as a paracancerous condition [19, 24, 25]. In the present study, 63 % of the M-CTACs were surrounded by mucosa without IM or with partial IM. These findings indicate that preceding IM is not necessary for the development of CTAC and support the concept of IM as a paracancerous condition rather than a precancerous lesion.
Endoscopic resection is an accepted treatment for early gastric cancers associated with a minimal risk of LNM. Gotoda et al. [6] reported that LNM was found in 2.2 % of M-cancers, 8.8 % of SM1-cancers, and 23.7 % of SM2-cancers. Furthermore, in M-cancer, the frequencies of LNM are higher in lesions with the following conditions: histologically poorly differentiated adenocarcinoma, presence of ulcerative changes, or positive for vessel permeation. In the present study, however, depth of invasion, ulcerative changes, and vessel permeation showed no correlation with LNM in CTAC. Therefore, for endoscopic treatment of CTAC, predictive factors for LNM other than these three factors should be considered.
Our results indicated that evaluations of the maximum diameter of PDC and the Ki-67 distribution pattern could be useful for distinguishing SM-cancer among CTACs (PDC >10 mm and Ki-67 expanded pattern), and for predicting LNM (PDC >20 mm and Ki-67 expanded pattern). In particular, prediction of LNM in patients with CTAC undergoing endoscopic resection is very important for the patients to consider whether additional surgical intervention is necessary. Accordingly, the combined use of these two findings–maximum diameter of PDC and the Ki-67 distribution pattern–can be expected to be effective for determining the treatment method for CTAC.
The frequency of p53 mutation in well-differentiated adenocarcinomas is higher than that in poorly differentiated adenocarcinomas. In previous reports, the frequency of p53 mutation in the early phase was 37–41 % in well-differentiated adenocarcinomas, and only 0–4 % in poorly differentiated adenocarcinomas [26–29]. These reports indicate that p53 mutation is an early event in tumorigenesis for well-differentiated adenocarcinoma, while the mutation is rare in poorly differentiated adenocarcinoma. Interestingly, none of the lesions examined in the present study showed p53 overexpression in the mucosal area, whereas only one lesion showed p53 overexpression in the submucosal invasive area. These results suggest that CTAC has characteristics similar to those of poorly differentiated adenocarcinomas in terms of p53 overexpression, despite the presence of tubular structures in the mucosal area.
The phenotypic expression of gastric adenocarcinomas was recently investigated using IHC for mucin and the brush border. For well- to moderately differentiated adenocarcinomas, Kawachi et al. [19] reported that 52 % of microscopic carcinomas with a maximum diameter of less than 3.0 mm showed neither a gastric nor an intestinal phenotype. The frequency of the gastric phenotype increased with the tumor size, and a phenotypic shift from the gastric to intestinal phenotype was observed with invasion into the submucosal layer or deeper [14, 30]. In the present study, all M-CTACs were classified as the mixed phenotype and of these, the MG-phenotype was predominant. The lesions in this study were larger than lesions in other studies [9, 14, 15, 19, 20]. This result suggested that CTAC may gradually acquire the gastric or intestinal phenotype during the lateral spread of the tumor into the mucosa. Consequently, CTAC shows heterogeneous phenotypic expression, even in the intramucosal phase. As compared with results in previously reported studies, the phenotypic expression of CTAC in this study was significantly different from that of the conventional histological types [9, 14, 15, 19, 20]. Our result indicates that CTAC is a distinct entity in terms of mucin and brush border expression.
On the other hand, we found various phenotypic expressions in the submucosal invasive area. This diversity might be explained by our results showing that the submucosal invasive component was significantly smaller than the intramucosal component in CTAC. Cancer cells in the submucosal invasive area were considered to be derived from a limited intramucosal area. Therefore, the phenotype of the cancer cells with invasive potential in the limited mucosal area might be reflected in the phenotype of the invasive area.
Based on the results of this study, we hypothesize the natural history of CTAC as follows: first, tumor tissue comprising mainly irregularly fused glands with low-grade cellular atypia develops and “crawls” horizontally in the mucosa; second, scattered foci of signet-ring cells appear to form PDC; third, the PDC area enlarges; and fourth, cancer cells invade the submucosa as poorly differentiated adenocarcinoma, with an infiltrative growth pattern and abundant stroma. In a review article by Kushima et al. [13] adenocarcinoma with characteristic glandular structures, called “shaking-hands structures”, which seems essentially identical to CTAC, was regarded as a “prediffuse type”. Our hypothesis may support their concept.
Morphologically, CTAC and conventional well- to moderately differentiated adenocarcinomas show similarities in having tubular components in the intramucosal area. Biologically, however, CTAC is similar to poorly differentiated adenocarcinomas in terms of the presence of signet-ring cells, the histological features of the submucosal invasive area, and the frequency of p53 overexpression. Further, based on the phenotypic classification, CTAC differs from the conventional histological types.
In conclusion, the use of the diagnostic term ‘CTAC’ has implications for selecting a distinct subgroup of gastric adenocarcinomas in the early phase. In CTAC, a larger PDC area and an expanded pattern of Ki-67-positive cells have practical implications for the prediction of submucosal invasion or LNM .
References
Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49.
Nakamura K. Histogenesis of gastric carcinoma and its clinicopathological significance. In: Nishi M, Ichikawa H, Nakajima T, et al., editors. Gastric Cancer. Tokyo: Springer; 1993. p. 112–32.
Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251–8.
Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Ishiguro S. Histological significance of foveolar type tubular adenocarcinoma of the stomach. Its histogenesis and relationship to undifferentiated carcinoma (in Japanese with English abstract). Med J Osaka Univ. 1987;39:507–15.
Saito A, Shimoda T, Nakanishi Y, Ochiai A, Toda G. Histologic heterogeneity and mucin phenotypic expression in early cancer. Pathol Int. 2001;51:165–71.
Takizawa T. Pathomorphology of the stomach (in Japanese). Tokyo: Igaku-shoin. 2003;168–72.
Takizawa T, Iwasaki Y, Maeda Y, Arai K, Kitamura M, Kato H, et al. Pathological problems concerning how to determine the resection-lines during operations for gastric cancer (in Japanese with English abstract). Stomach Intestine. 1990;25:319–28.
Kato Y, Yanagisawa A, Sugano H. Biopsy interpretation in diagnosis of gastric carcinoma. In: Nishi M, Ichikawa H, Nakajima T, et al., editors. Gastric Cancer. Tokyo: Springer; 1993. p. 133–50.
Kushima R, Vieth M, Borchard F, Stolte M, Mukaisho K, Hattori T. Gastric-type well-differentiated adenocarcinoma and pyloric gland adenoma of the stomach. Gastric Cancer. 2006;9:177–84.
Egashira Y, Shimoda T, Ikegami M. Mucin histochemical analysis of minute gastric differentiated adenocarcinoma. Pathol Int. 1999;49:55–61.
Mizoshita T, Tsukamoto T, Inada K, Ogasawara N, Hirata A, Kato S, et al. Immunohistochemically detectable Cdx2 is present in intestinal phenotypic elements in early gastric cancers of both differentiated and undifferentiated types, with no correlation to non-neoplastic surrounding mucosa. Pathol Int. 2004;54:392–400.
Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, et al. Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol. 2003;129:727–34.
Fukunaga M, Monden T, Nakanishi H, Ohue M, Fukuda K, Tomita N, et al. Immunohistochemical study of p53 in gastric carcinoma. Am J Clin Pathol. 1994;101:177–80.
Gabbert HE, Müller W, Schneiders A, Meier S, Hommel G. The relationship of p53 expression to the prognosis of 418 patients with gastric carcinoma. Cancer. 1995;76:720–6.
Kawachi H, Takizawa T, Eishi Y, Shimizu S, Kumagai J, Funata N, et al. Absence of either gastric or intestinal phenotype in microscopic differentiated gastric carcinomas. J Pathol. 2003;199:436–46.
Koseki K, Takizawa T, Koike M, Ito M, Nihei Z, Sugihara K. Distinction of differentiated type early gastric carcinoma with gastric type mucin expression. Cancer. 2000;89:724–32.
Ninomiya Y, Yanagisawa A, Kato Y, Tomimatsu H. Unrecognizable intramucosal spread of diffuse-type mucosal gastric carcinomas of less than 20 mm in size. Endoscopy. 2000;32:604–8.
Jass JR. Role of intestinal metaplasia in the histogenesis of gastric carcinoma. J Clin Pathol. 1980;33:801–10.
Filipe MI, Potet F, Bogomoletz WV, Dawson PA, Fabiani B, Chauveinc P, et al. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer: preliminary data from prospective study from three centers. Gut. 1985;26:1319–26.
Matsukuma A, Mori M, Enjoji M. Sulphomucin-secreting intestinal metaplasia in the human gastric mucosa: an association with intestinal-type gastric carcinoma. Cancer. 1990;66:689–94.
Tatematsu M, Ichinose M, Miki K, Hasegawa R, Kato T, Ito N. Gastric and intestinal phenotype expression of human stomach cancers as revealed by pepsinogen immunohistochemistry and mucin histochemistry. Acta Pathol Jpn. 1990;40:494–504.
Ranzani GN, Luinetti O, Padovan LS, Calistri D, Renault B, Burrel M, et al. p53 gene mutations and protein nuclear accumulation are early events in intestinal type gastric cancer but late events in diffuse type. Cancer Epidemiol Biomarkers Prev. 1995;4:223–31.
Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192–8.
Tamura G, Sato K, Akiyama S, Tsuchiya T, Endoh Y, Usuba O, et al. Molecular characterization of undifferentiated-type gastric carcinoma. Lab Invest. 2001;81:593–8.
Uchino S, Noguchi M, Ochiai A, Saito T, Kobayashi M, Hirohashi S. p53 mutation in gastric cancer: a genetic model for carcinogenesis is common to gastric and colorectal cancer. Int J Cancer. 1993;54:759–64.
Yoshikawa A, Inada K, Yamachika T, Shimizu N, Kaminishi M, Tatematsu M. Phenotypic shift in human differentiated gastric cancers from gastric to intestinal epithelial cell type during progression. Gastric Cancer. 1998;1:134–41.
Acknowledgments
The authors earnestly thank Dr. Toichiro Takizawa, former professor of Tokyo Medical and Dental University, both for providing the idea for this study and for his sincere encouragement.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okamoto, N., Kawachi, H., Yoshida, T. et al. “Crawling-type” adenocarcinoma of the stomach: a distinct entity preceding poorly differentiated adenocarcinoma. Gastric Cancer 16, 220–232 (2013). https://doi.org/10.1007/s10120-012-0173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0173-2