Abstract
In this study, we developed a new method to separate terpenoids from byproducts discharged during sugi wood-drying process by solvent-assisted-distillation (SAD). First, the sample was distillated from a mixed solvent, of water and triethylene glycol. Then, the volatile compounds in the sample were fractionated sequentially according to boiling point. Using SAD, we separated compounds like sesuquiterpenes from the water distillates (WD) fraction by conventional distillation methods. In addition, high boiling point compounds, such as diterpenes, were separated from the triethylene glycol distillates (TGD) fraction. Based on gas chromatography/mass spectrometry (GC/MS) analysis, the main components of the byproducts were sandaracopimarinol (18.74 %), ferruginol (16.45 %), 6,7-dehydroferruginol (7.97 %), abietadiene (6.39 %), and sandaracopimarinal (6.08 %). We purified ferruginol from these sugi wood-drying byproducts using SAD and chromatography; the yield was 5.3 % and the purity was 99.8 %.
Similar content being viewed by others
Introduction
Cryptomeria japonica (sugi) is one of the most common conifers in Japan. C. japonica has been employed by people to produce building materials, laminated wood, and veneer sheets for plywood. Numerous studies have investigated C. japonica for a long time. Essential oils obtained from C. japonica have been shown to exhibit antifungal activity [1], anti-termite activity [2], anti-mosquito activity [3], inhibitory activity against Skeletonema costatum [4], relaxation effects [5], and cytotoxic activity [6].
Essential oils obtained from many plants have been used for food, medicine, perfumes and cosmetics all over the world [7]. Numerous volatile organic compounds, like terpenoids, are contained in essential oils.
There are traditional methods to extract terpenoids industrially, for example, hydrodistillation, steam distillation, solvent extraction, and supercritical extraction. In recent years, new distillation methods, such as microwave-assisted hydrodistillation [8], and vacuum microwave-assisted steam distillation [9] have been developed for various purposes.
At the manufacturing process of plywood, green veneer sheets made of sugi wood are dried by heating around 170 °C. The steam evolved in the wood-drying process is recovered in tanks by cooling. The steam is then condensed into water by cooling, and the precipitate in the tanks has a high viscosity, like tar. This precipitate is used as fuel for the plywood factories. However, it has been reported that the byproducts discharged during sugi wood-drying process (BWP) are rich in terpenoids originating from sugi wood, such as sandaracopimarinol, ferruginol, 6,7-dehydroferruginol [10]. In this study, we developed a new method for separating terpenoids from the BWP by solvent-assisted-distillation (SAD). Ferruginol was purified from the distillate by conventional chromatography. Thus, the aims of this study were to separate terpenoids by SAD, to purify ferruginol more easily, and to add value to BWP, all of which can be applied to the wood industry.
Materials and methods
Materials
Reference sample
Ferruginol was deposited at the Faculty of Agriculture, Iwate University [11]. The vial was sealed and kept at −20 °C in the dark until use.
Plant material
Sawdust meal of sugi heartwood was provided by Kimura-sangyou Co., Ltd. (Iwate, Japan). The sawdust was divided into subsamples and stored at −30 °C until use. The water content was 38.5 %. This sawdust was used for steam distillation and solvent extraction.
BWP
BWP, the byproducts discharged from sugi wood-drying process, was obtained from Nisshin Co., Ltd. (Shimane, Japan). The bottle used to preserve BWP was capped tightly and kept in the dark at 4 °C until use. BWP was used for SAD. Prior to gas chromatography/mass spectrometry (GC/MS) and gas chromatography/flame ionization detection (GC/FID) analysis, BWP was dissolved in ethyl acetate, was dried over anhydrous sodium sulfate, and n-heptadecane was added as internal standard. The water content of BWP used in this study was 24.2 % (w/w).
Chemicals
All chemicals were reagent grade. Triethylene glycol, palladium-activated carbon (10 % Pd), Wakogel C-300HG (40–60 μm), and n-heptadecane, were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Silica gel 60G was obtained from Merck (Darmstadt, Germany). Cellulose powder (40–100 mesh) was obtained from Toyo Roshi Kaisha., Ltd. (Tokyo, Japan). BSTFA + 10 % TMCS (No. 38840) was obtained from Thermo Fisher Scientific K.K., (Yokohama, Japan). Chloroform-d (99.8 % atom % D, containing 0.03 % (v/v) TMS), used for nuclear magnetic resonance (NMR) analysis, was obtained from Sigma-Aldrich (Tokyo, Japan).
Distillation
Steam distillation
Air-dried sawdust (150 g) of sugi was steam-distilled for 7 h. The distillate containing the volatile organic compounds obtained from the sample was collected using a condenser. Liquid phase extraction was then performed under a 1:1 (v/v) ratio of n-hexane/water. Anhydrous sodium sulfate was then completely dissolved in the organic fraction sufficiently. After filtration, the volatile organic compounds were concentrated and the remaining solvent removed by blowing with a stream of N2 gas. The volatile organic compounds coming from the steam distillates (SD) were dissolved in n-hexane. n-Heptadecane was added as internal standard and the mixture was subjected to analysis by GC/MS (Shimadzu, Japan) and GC/FID (Shimadzu, Japan).
Solvent extraction
For solvent extraction, a Soxhlet-extraction apparatus was used. An 80 g portion of air-dried sample was extracted with acetone for 7 h. The water in the acetone extract was removed by adding ethyl ether and saturated saline solution (acetone extract/diethyl ether/saturated saline solution = 1:1:2). The organic fraction was dried over anhydrous sodium sulfate and the solvent extracts (SE) were concentrated, and the compounds in the SE were determined as described above. The compounds obtained by solvent extraction were dissolved in acetone, n-heptadecane was added as internal standard, and then they were analyzed by GC/MS and GC/FID.
SAD
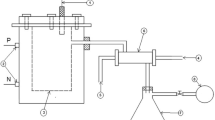
We selected an apparatus typically used for hydrodistillation. A 240 g portion of BWP was mixed with water (1200 g) and triethylene glycol (1200 g) in a separable flask. The flask was heated using a mantle heater at 100 V. The temperature was recorded during distillation. First, a boiling point was reached at 100 °C, and the water distillates (WD) fraction was distilled and collected using a condenser. Then triethylene glycol (1200 g) was added to the separation flask. A second, boiling point was reached at 285 °C, and the triethylene glycol distillates (TGD) fraction was distilled and collected through the condenser. The distillation time for the WD fraction was 3 h, and for the TGD fraction was 3.5 h. The total volume of the WD fraction was 1160 ml, and of the TGD fraction was 2200 ml.
Each fraction collected was liquid-phase extracted. The WD fraction was mixed with the same amount of n-hexane (1:1, v/v). The TGD fraction was mixed with the same amount of water and twice the amount of n-hexane (1:1:2, by volume), and fractionated a total of three times under the same conditions. Each organic layer was dried over anhydrous sodium sulfate. After filtration, the distilled compounds in the WD fraction were determined in the same manner using N2 gas, and the distilled compounds in the TGD fraction were determined after drying under reduced pressure.
Purification of ferruginol
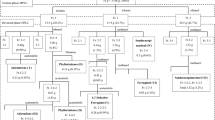
The purification process for ferruginol is shown in Fig. 1. The organic layer from the TGD fraction was concentrated and purified by silica gel column chromatography using n-hexane/diethyl ether, 9:1, (v/v), as eluent. The eluted fraction was separated by medium pressure liquid chromatography (MPLC) using a normal-phase column (Yamazen ULTRAPACK SI-40 50 mm i.d. × 300 mm; mobile phase: n-hexane/ethyl ether, 7:3, (v/v); ShodexRI-70, detector; EYELA VSP-3050, pump; flow rate 10 ml/min) and the eluted compounds were then divided into fractions F1 through F8. To convert 6,7-dehydroferruginol to ferruginol, the F3 fraction was catalytically reduced. The reaction product (F3′) was poured into a separable funnel containing cellulose powder (40–100 mesh) and Wakogel C-300HG (40–60 μm), and filtered by using an aspirator. Finally, the F3′ fraction was divided into F3-1 through F3-3 by MPLC with a reversed-phase column (Yamazen ULTRAPACK ODS-SM-50 50 mm i.d. × 300 mm, mobile phase 100 % methanol, with the same detector, pump, and flow rate used for normal-phase column chromatography). The purified ferruginol was in F3-3.
GC/MS analysis
GC/MS was carried out using a GCMS-QP2010 system (Shimadzu, Japan) set up with an HP-5 column (30 mm × 0.25 mm i.d., 0.25 μm film thickness, Agilent, USA). Helium was used as the carrier gas at a flow rate of 1.61 ml/min. The initial oven temperature was 60 °C, and it was increased at 3.0 °C/min to 240 °C. The injector temperature was 250 °C and the samples (1 μl) were injected using a 1:40 split ratio. Mass spectra were taken at 70 eV and the ion source temperature was 250 °C. The ionization method was EI and the mass range was from 40 to 500 m/z.
GC/FID analysis
GC/FID was conducted using a GC-1700 system (Shimadzu, Japan) attached to a Chromatopac C-R7A plus recorder (Shimadzu, Japan). An HP-5 column was used for this analysis. The flame ionization detector temperature was 250 °C and N2 was used as the carrier gas at a flow rate of 1.3 ml/min. Other parameters were set as for GC/MS analysis.
NMR spectrum analysis
NMR spectra were recorded on a JEOL AL400 FT-NMR spectrometer. Purified ferruginol (50 mg) was dissolved in 0.7 ml chloroform-d. The NMR spectral data were identified by comparison with data in the literature [12, 13].
Ferruginol: 1H NMR (CDCl3, 400 MHz) δ (ppm) = 0.91 (3H,s,H-18), 0.93 (3H, s, H-19), 1.16 (3H, s, H-20), 1.22 (1H, d, J = 6.8 Hz, H-16), 1.24 (1H, d, J = 6.8 Hz, H-17), 2.76 (1H, ddd, J = 21.4, 10.2, 6.3 Hz, H-7β), 2.85 (1H, ddd, J = 16.0, 6.2, 1.6 Hz, H-7α), 3.11 (1H, sept, J = 6.8 Hz, H-15), 4.68 (s, OH), 6.62 (1H, s, H-11), 6.83 (1H, s, H-14); 13CNMR (CDCl3, 100 MHz), δ (ppm) = 19.2, 19.3, 21.6, 22.6, 22.8, 24.8, 26.8, 29.8, 33.3, 33.4, 37.5, 38.8, 41.7, 50.3, 111.0, 126.6, 127.3, 131.4, 148.6, 150.6.
Results and discussion
Chemical composition of volatile organic compounds
Samples from steam distillation, solvent extraction, and BWP were qualitatively analyzed by GC/MS. Volatile organic compounds were identified by comparing their relative retention times and mass spectra with those in the literature [14] and/or the NIST Mass Spectral Library (Table 1).
In all, 29 compounds were identified in SD, SE, and BWP. These compounds, which constituted 91.95, 85.38, and 91.37 % of all GC peaks in SD, SE, and BWP, respectively, were classified into several terpenoid groups: sesquiterpene hydrocarbons, oxygenated sesquiterpenes, diterpene hydrocarbons, and oxygenated diterpenes.
In SD, 21 compounds, constituting 91.95 % of total GC peaks, could be identified. The major fractions were oxygenated sesquiterpenes (49.42 %) and sesquiterpene hydrocarbons (34.74 %). The major components of SD were δ-cadinene (20.79 %), 1-epi-cubenol (9.93 %), cubenol (5.87 %), α-eudesmol (5.44 %), and cryptomerione (3.59 %). Saito et al. [15] reported that the main component of C. japonica wood oil obtained by steam distillation is δ-cadinene (30.45 %).
In SE, 22 compounds were identified, constituting 85.38 % of total GC peaks. Oxygenated diterpenes (42.90 %) and oxygenated sesquiterpenes (35.81 %) were the predominant constituents of SE, and included cubebol (17.07 %), ferruginol (14.24 %), sandaracopimarinol (13.89 %), 6,7-dehydroferruginol (10.13 %), and epi-cubebol (6.23 %). Cubebol and epi-cubebol have mosquito larvicidal activities [16], and cubenol, epi-cubenol, cubebol are termiticidal [17]. Noting the quantity of these compounds in SD and SE, it was suggested that cubebol could be dehydrated and changed to δ-cadinene [18]. Nagahama and Tazaki [19] reported that the main components of C. japonica wood oils obtained by solvent extraction are ferruginol (13.5–30.8 %) and sandaracopimarinol (12.3–32.1 %).
In BWP, 22 compounds, constituting 91.37 % of the total peaks, were identified. Oxygenated sesquiterpenes and oxygenated diterpenes were the most abundant constituents (21.48 and 58.10 %, respectively). The main constituents of BWP were sandaracopimarinol (18.74 %), ferruginol (16.45 %), 6,7-dehydroferruginol (7.97 %), abietadiene (6.39 %), and sandaracopimarinal (6.08 %). Sandaracopimarinol has antimicrobial activity [20] and termiticidal activity [21]. Ferruginol also shows antimicrobial activity [13, 20], antioxidant activity [22], gastroprotective and ulcer healing effects [23], and termiticidal activity [24]. Sandaracopimarinol and ferruginol show synergistic inhibition of shiitake mycelial growth [25]. 6,7-Dehydroferruginol is a compound related to ferruginol, and has antifungal activity [26]. Abietadiene shows an effect on human electroencephalograms [27]. This chemical composition is typical of BWP. Interestingly, oxygenated diterpenes consisted of approximately 60 % of this fraction. The types of components were similar to those of SE.
Purification of volatile organic compounds from BWP
We developed a new distillation method, referred to as the solvent-assisted distillation or SAD, to purify the volatile organic compounds contained in BWP. Water and triethylene glycol were mixed and used for the distillation as solvent. Sesquiterpenes and diterpenes were separated in the WD fraction and the TGD fraction, depending on the boiling point of the solvent. As shown in Fig. 2 and Table 2, sesquiterpenes and diterpenes contained in BWP were divided into two fractions by SAD, implying that this method is well suited to separating volatile compounds that have high boiling points.
In the WD fraction, 24 compounds were identified in total, constituting 96.24 % of GC peaks. Sesquiterpene hydrocarbons (56.38 %), and oxygenated sesquiterpenes (26.90 %) were the main components of the WD fraction. The major components were δ-cadinene (30.57 %), 1-epi-cubenol (6.93 %), dauca-5,8-diene (6.10 %), trans-muurola-4(14),5-diene (5.99 %), and cubenol (5.44 %). After distillation, the contents of epi-cubebol and cubebol content decreased, whereas that of δ-cadinene increased (Table 2). From this result, it was suggested that dehydration occurred during distillation, like the previous study of steam distillation [18].
In the TGD fraction, 19 compounds constituting 92.74 % of the GC peaks were identified. Diterpene hydrocarbons (20.72 %) and oxygenated diterpenes (57.08 %) were the main categories. The principal constituents were sandaracopimarinol (26.75 %), ferruginol (19.68 %), isophyllocladene (7.96 %), abietadiene(6.70 %), 6,7-dehydroferruignol (5.51 %), and sandaracopimarinal (5.14 %). Isophyllocladene was not included in BWP. Similarly, some compounds that were not in the BWP were found in the WD and TGD fractions, but it is not clear why this occurred. The total yield, including the WD fraction (23.5 g, 9.8 %) and TGD fraction (113.1 g, 47.1 %), was 56.9 % of BWP (240 g). Most of the ferruginol contained in BWP was recovered in the TGD fraction by SAD. Kano et al. [24] reported that the amount of ferruginol was maintained after high-temperature drying.
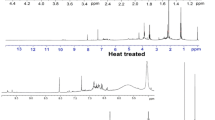
Ferruginol is considered to have high commercial potential because of its various bioactivities. We attempted to conduct large-scale purification of ferruginol using MPLC for such industrial applications. The purification steps and yield of ferruginol are shown in Table 3. After preliminary purification using a silica gel column, the TGD obtained by SAD from BWP were separated by normal-phase MPLC. In the following step, 6,7-dehydroferruginol, which is difficult to separate from ferruginol, was converted into ferruginol by catalytic reduction (Fig. 3). Finally, reversed-phase MPLC was performed, and ferruginol was purified from the original BWP at a yield of 5.3 %. As mentioned above, ferruginol exhibits several biological activities, including antimicrobial activity and antioxidant activity. However, since the basis of these activities remains unclear, an investigation of these activities should be undertaken in the future.
References
Cheng SS, Lin HY, Chang ST (2005) Chemical composition and antifungal activity of essential oils from different tissues of Japanese cedar (Cryptomeria japonica). J Agric Food Chem 53:614–619
Cheng SS, Chang HT, Wu CL, Chang ST (2007) Anti-termitic activities of essential oils from coniferous trees against Coptotermes formosanus. Bioresource Technol 98:456–459
Gu HJ, Cheng SS, Lin CY, Huang CG, Chen WJ, Chang ST (2009) Repellency of essential oils of Cryptomeria japonica (Pinaceae) against adults of the mosquitoes Aedes aegypti and Aedes albopictus (Diptera:Culicidae). J Agric Food Chem 57:11127–11133
Tsuruta K, Yoshida Y, Kusumoto N, Sekine N, Ashitani T, Takahashi K (2011) Inhibition activity of essential oils obtained from Japanese trees against Skeletonema costatum. J Wood Sci 57:520–525
Cheng WW, Lin CT, Chu FH, Chang ST, Wang SY (2009) Neuropharmacological activities of phytoncide released from Cryptomeria japonica. J Wood Sci 55:27–31
Cha JD, Kim JY (2012) Essential oil from Cryptomeria japonica induces apoptosis in human oral epidermoid carcinoma cells via mitochondrial stress and activation of caspases. Molecules 17:3890–3901
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Özek G, Demirci F, Özek T, Tabanca N, Wedge DE, Khan SI, Başer KHC, Duran A, Hamzaoglu E (2010) Gas chromatographic–mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. J Chromatogr A 1217:741–748
Ohira T, Matsui N, Kaneko T, Tanaka Y (2010) Efficient extraction of essential oil from woody materials using vaccume microwave assisted steam distillation (in Japanese). Aroma Res 11:148–155
Ohira T (2009) Efficient utilisation of condensed water discharged from wood-drying processes (in Japanese). J Jpn Associ Odor Environ 40:400–411
Kofujita H, Fujino Y, Sasaki T, Hasebe M, Ota M, Suzuki K (2001) Antifungal activity of the bark of Cryptomaria japonica and its relevant components (in Japanese). Mokuzai Gakkaishi 47:479–486
Tezuka Y, Kasimu R, Li JX, Basnet P, Tanaka K, Namba T, Kadota S (1998) Constituents of roots of Salvia deserta SCHANG (Xinjiang-Danshen). Chem Pharm Bull 46:107–112
Li WH, Chang ST, Chang SC, Chang HT (2008) Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat Prod Res 22:1085–1093
Adams RP (2012) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Books, Illinois
Saito K, Okabe T, Fukui T, Iinuma K (1995) Antibacterial activities of essential oil extracted from Thujopsis dolabrata var. hondae, Chamaecyparis obtusa, and Cryptomeria japonica D. Don against methicillin-resistant Streptococcus aureus (in Japanese). Aromatopia 10:49–52
Gu HJ, Cheng SS, Huang CG, Chen WJ, Chang ST (2009) Mosquito larvicidal activities of extractives from black heartwood-type Cryptomeria japonica. Parasitol Res 105:1455–1458
Arihira S, Umeyama A, Bando S, Kobuke S, Imoto S, Ono M, Yoshikawa K, Amita K, Hashimoto S (2004) Termicidal constituents of the black-heart of Cryptomeria japonica (in Japanese). Mokuzai Gakkaishi 50:413–421
Matsushita Y, Sugamato K, Miyakubo K, Kurogi C, Matsui T, Oda H, Fujimoto H (2008) Chemical changes in terpenes of sugi (Cryptomeria japonica) wood during steam drying in kiln at high temperature. J Wood Sci 54:476–482
Nagahama S, Tazaki M (1993) Terpenoids of wood oil of sugi (Cryptomeria japonica) peculiarities of obisugi variety (in Japanese). Mokuzai Gakkaishi 39:1077–1083
Matushita Y, Hwang YH, Sugamoto K, Matsui T (2006) Antimicrobial activity of heartwood components of sugi (Cryptomeria japonica) against several fungi and bacteria. J Wood Sci 52:552–556
Sogabe A, Kinjo K, Abe F, Yamauchi T, Yaga S (2000) Termicidal substances from the heartwood of Cryptomeria japonica D. Don (in Japanese). Mokuzai Gakkaishi 46:124–131
Wang SY, Wu JH, Shyur LF, Kuo YH, Chang ST (2002) Antioxidant activity of abietane-type diterpens from heartwood of Taiwania cryptomerioides Hayata. Holzforschung 56:487–492
Rodríguez JA, Theoduloz C, Yáñez T, Becerra J, Schmeda-Hirschmann G (2006) Gastroprotective and ulcer healing effect of ferruginol in mice and rats :assessment of its mechanism of action using in vitro models. Life Sci 78:2503–2509
Kano H, Shibutani S, Hayashi K, Iijima Y, Doi S (2004) Effect of high-temperature drying processes on termite resistance of sugi (Cryptomeria japonica) heartwood (in Japanese). Mokuzai Gakkaishi 50:91–98
Matui T, Matsushita Y, Sugamoto K, Ogawa K, Komiyama A, Muta S (2001) Mycelial growth inhibition of shiitake (Lentinula edodes) by several terpenoids isolated from sugi (Cryptomeria japonica) wood (in Japanese). Mokuzai Gakkaishi 47:58–62
Kusumoto N, Ashitani T, Murayama T, Ogiyama K, Takahashi K (2010) Antifungal abietane-type diterpenes from the cones of Taxodium distichum rich. J Chem Ecol 36:1381–1386
Sakamaki S, Kofujita H, Sugawara M (2013) Effect of odors from Cryptomeria japonica essential oils on human electroencephalogram (in Japanese). Aroma Res 14:64–71
Acknowledgments
We are very grateful to Nisshin Co., Ltd. and Kimura-sangyou Co., Ltd. for supplying BWP and sugi wood samples.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tsujimura, M., Tsuji, M., Kofujita, H. et al. New separation method for terpenoids in byproducts discharged during sugi wood-drying process, and purification of ferruginol. J Wood Sci 61, 308–315 (2015). https://doi.org/10.1007/s10086-015-1471-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-015-1471-1