Abstract
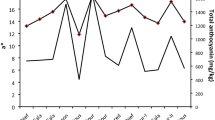
In order to obtain basic data required for utilization of guava leaf as a functional substance, the antioxidant activities of aqueous extracts from 3 cultivars of guava leaf (‘Apple color’, ‘Ruby’, and ‘Safeda’) were examined. The total phenolic contents of the aqueous extracts ranged from 257.38 to 293.25 mg/g gallic acid equivalents. DPPH, ABTS, reducing power, ferric reducing antioxidant power (FRAP), ferric thiocyanate (FTC), and malondialdehyde (MDA) assays indicated that the aqueous extract of the ‘Ruby’ cultivar was the most potent radicalscavenger and reducing agent compared to the other 2 cultivars. Therefore, this study verified that aqueous extract from the ‘Ruby’ cultivar possessed strong antioxidant activity that correlated to its high level of phenolics, particularly gallic acid. In conclusion, the aqueous extract of the ‘Ruby’ cultivar of guava leaf may be utilized as an effective source of functional food materials, including natural antioxidants.
Similar content being viewed by others
References
Singh A. Chemical and biochemical aspects of activated oxygen: Singlet oxygen, superoxide anion, and related species. Vol. I, pp. 17–28. In: Handbook of Free Radical and Antioxidant in Biomedicine. Miquel J, Quintaanilha AT, Weber H (eds). CRC Press, Inc., Boca Raton, FL, USA (1989)
Aruoma O. Assessment of potential prooxidant and antioxidant actions. J. Am. Oil Chem. Soc. 73: 1617–1625 (1996)
Sawa T, Akaike T, Maeda H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J. Biol. Chem. 275: 32467–32474 (2000)
Choi HR, Choi JS, Han YN, Bae SJ, Chung HY. Peroxynitrite scavenging activity of herb extracts. Phytother. Res. 16: 364–367 (2002)
Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radical Bio. Med. 25: 392–403 (1998)
Cao G, Sofic E, Prior RL. Antioxidant capacity of tea and common vegetables. J. Agr. Food Chem. 44: 3426–3431 (1996)
Branen A. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 52: 59–63 (1975)
Joseph B, Priya M. Review on nutritional, medicinal, and pharmacological properties of guava (Psidium Guajava Linn.). Int. J. Pharma. Bio. Sci. 2: 53–69 (2011)
Morton JF. Fruits of Warm Climates. Julia F Morton, Miami, FL, USA. pp. 356–363. (1987)
Oh WK, Lee CH, Lee MS, Bae EY, Sohn CB, Oh H, Kim BY, Ahn JS. Antidiabetic effects of extracts from Psidium guajava. J. Ethnopharmacol. 96: 411–415 (2005)
Okuda T, Yoshida T, Hatano T, Yazaki K, Ikegami Y, Shingu T. Guavins A, C, and D, complex tannins from Psidium guajava. Chem. Pharm. Bull. 35: 443–446 (1987)
Begum S, Hassan SI, Siddiqui BS, Shaheen F, Nabeel Ghayur M, Gilani AH. Triterpenoids from the leaves of Psidium guajava. Phytochemistry 61: 399–403 (2002)
Meckes M, Calzada F, Tortoriello J, González JL, Martínez M. Terpenoids isolated from Psidium guajava hexane extract with depressant activity on central nervous system. Phytother. Res. 10: 600–603 (1996)
Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo C, Arnason JT. Quercetin glycosides in Psidium guajava L. leaves and determination of a spasmolytic principle. Arch. Med. Res. 25: 11–15 (1994)
Tanaka T, Ishida N, Ishimatsu M, Nonaka G, Nishioka I. Tannins and related compounds. CXVI. Six new complex tannins, guajavins, psidinins, and psiguavin from the bark of Psidium guajava L. Chem. Pharm. Bull. 40: 2092–2098 (1992)
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 19: 669–675 (2006)
Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, Sumal Saraya ML, Ruangsomboon O. Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. J. Ethnopharmacol. 67: 203–212 (1999)
Hsieh CL, Lin YC, Yen GC, Chen HY. Preventive effects of guava (Psidium guajava L.) leaves and its active compounds against α-dicarbonyl compounds-induced blood coagulation. Food Chem. 103: 528–535 (2007)
Wang H, Du YJ, Song HC. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 123: 6–13 (2010)
Chen HY, Yen GC. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 101: 686–694 (2007)
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 181: 1199–1200 (1958)
Fellegrini N, Ke R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. pp. 379–389. In: Methods in Enzymology. Lester P (ed). Academic Press, New York, NY, USA (1999)
Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 44: 307–315 (1986)
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239: 70–76 (1996)
Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from acacia confusa bark and heartwood. J. Agr. Food Chem. 49: 3420–3424 (2001)
Jeong CH, Jeong HR, Choi SG, Shim KH, Heo HJ. Neuronal cell protection and antioxidant activities of hot water extract from commercial buckwheat tea. Korean J. Food Preserv. 18: 358–365 (2011)
Pyo YH, Lee TC, Logendra L, Rosen RT. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 85: 19–26 (2004)
Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agr. Food Chem. 51: 6657–6662 (2003)
Sevanian A, Ursini F. Lipid peroxidation in membranes and lowdensity lipoproteins: Similarities and differences. Free Radical Bio. Med. 29: 306–311 (2000)
Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agr. Food Chem. 50: 3713–3717 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, C.H., Bae, Y.I., Park, S.J. et al. Antioxidant activities of aqueous extracts from three cultivars of guava leaf. Food Sci Biotechnol 21, 1557–1563 (2012). https://doi.org/10.1007/s10068-012-0207-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-012-0207-x