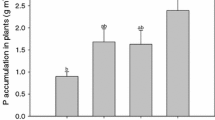
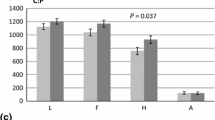
Abstract
Soil mineral weathering may serve as a sink for atmospheric carbon dioxide (CO2). Increased weathering of soil minerals induced by elevated CO2 concentration has been reported previously in temperate areas. However, this has not been well documented for the tropics and subtropics. We used model forest ecosystems in open-top chambers to study the effects of CO2 enrichment alone and together with nitrogen (N) addition on inorganic carbon (C) losses in the leachates. Three years of exposure to an atmospheric CO2 concentration of 700 ppm resulted in increased annual inorganic C export through leaching below the 70 cm soil profile. Compared to the control without any CO2 and N treatments, net biocarbonate C (HCO3 −-C) loss increased by 42%, 74%, and 81% in the high CO2 concentration treatment in 2006, 2007, and 2008, respectively. Increased inorganic C export following the exposure to the elevated CO2 was related to both increased inorganic C concentrations in the leaching water and the greater amount of leaching water. Net annual inorganic C (HCO3 −-C and carbonate C: CO3 2−-C) loss via the leaching water in the high CO2 concentration chambers reached 48.0, 49.5, and 114.0 kg ha−1 y−1 in 2006, 2007, and 2008, respectively, compared with 33.8, 28.4, and 62.8 kg ha−1 y−1 in the control chambers in the corresponding years. The N addition showed a negative effect on the mineral weathering. The decreased inorganic C concentration in the leaching water and the decreased leaching water amount induced by the high N treatment were the results of the adverse effect. Our results suggest that tropical forest soil systems may be able to compensate for a small part of the atmospheric CO2 increase through the accelerated processing of CO2 into HCO3 −-C during soil mineral weathering, which might be transported in part into ground water or oceans on geological timescales.






Similar content being viewed by others
References
Andrews JA, Schlesinger WH. 2001. Soil CO2 dynamics, acidification, and chemical weathering in a temperate forest with experimental CO2 enrichment. Global Biogeochem Cycles 15(1):149–62.
Berner RA, Kothavala Z. 2001. GEOCARB III: a revised model of atmospheric CO2 over hanerozoic time. Am J Sci 301:182–204.
Bernhardt ES, Barber JJ, Pippen JS, Taneva L, Andrews JA, Schlesinger WH. 2006. Long-term effects of free air CO2 enrichment (FACE) on soil respiration. Biogeochemistry 77:91–116.
Bunce JA. 2004. Carbon dioxide effects on stomatal responses to the environment and water use by crops under field conditions. Oecologia 140:1–10.
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ. 2000. Acceleration of global warming due to carbon-cycle feedbacks in a coupled model. Nature 408:184–7.
de Graaff M, van Groenigen K, Six J, van Kessel C. 2006. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–91.
Deng Q, Zhou GY, Liu JX, Liu SZ, Duan HL, Zhang DQ. 2010. Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosciences 7:315–28.
Duan HL, Liu JX, Deng Q, Chen XM, Zhang DQ. 2009. Interactive effects of elevated CO2 and N addition on plant biomass accumulation and allocation in subtropical forest ecosystems: a mesocosm study. Chin J Plant Ecol 33:570–9 (in Chinese with English abstract).
Gilfillan SMV, Lollar BS, Holland G, Blagburn D, Stevens S, Schoell M, Cassidy M, Ding Z, Zhou Z, Lacrampe-Couloume G, Ballentine CJ. 2009. Solubility trapping in formation water as dominant CO2 sink in natural gas fields. Nature 458:614–18.
Gill RA, Polley HW, Johnson HB, Anderson LJ, Maherali H, Jackson RB. 2002. Nonlinear grassland responses to past and future atmospheric CO2. Nature 417:279–82.
Hagedorn F, Spinnler D, Bundt M, Blaser P, Siegwolf R. 2003. The input and fate of new C in two forest soils under elevated CO2. Glob Change Biol 9:862–73.
King JS, Pregitzer KS, Zak DR, Sober J, Isebrands JG, Dickson RE, Hendrey GR, Karnosky DF. 2001. Fine-root biomass and fluxes of soil carbon in young stands of paper birch and trembling aspen as affected by elevated atmospheric CO2 and tropospheric O3. Oecologia 128:237–50.
Kripalani RH, Oh JH, Chaudhari HS. 2007. Response of the East Asian summer monsoon to doubled atmospheric CO2: coupled climate model simulations and projections under IPCC AR4. Theor Appl Climatol 87:1–28.
Lichter J, Barron SH, Bevacqua CE, Finzli AC, Irving KE, Stemmler EA, Schlesinger WH. 2005. Soil carbon sequestration and turnover in a pine forest after six years of atmospheric CO2 enrichment. Ecology 86:1835–47.
Liu JX, Zhang DQ, Zhou GY, Faivre-Vuillin B, Deng Q, Wang CL. 2008. CO2 enrichment increases nutrient leaching from model forest ecosystems in subtropical China. Biogeosciences 5:1783–95.
Luo Y, Hui D, Zhang D. 2006. Elevated carbon dioxide stimulates net accumulations of carbon and nitrogen in terrestrial ecosystems: a meta-analysis. Ecology 87:53–63.
Mo JM, Brown S, Xue JH, Fang YT, Li ZA. 2006. Response of litter decomposition to simulated N deposition in 18 disturbed, rehabilitated and mature forests of subtropical China. Plant Soil 282:135–51.
Morgan JA, Pataki DE, Korner C, Clark H, Del Grosso SJ, Grunzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JB, Nowak RS, Parton WJ, Polley HW, Shaw MR. 2004. Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25.
Nelson DW, Sommers LE. 1982. Carbon and organic matter. In: Page AL, Mille RH, Keeney DR, Eds. Methods of soil analysis: part two. Chemical and microbiological properties. Madison, Wisconsin: American Society of Agronomy. p 561–79.
Nelson JA, Morgan JA, LeCain DR, Mosier AR, Milchunas DG, Parton BA. 2004. Elevated CO2 increases soil moisture and enhances plant water relations in a long-term field study in semi-arid shortgrass steppe of Colorado. Plant Soil 259:169–79.
Niklaus PA, Spinnler D, Kornerb C. 1998. Soil moisture dynamics of calcareous grassland under elevated CO2. Oecologia 117:201–8.
Oh NH, Raymond PA. 2006. Contribution of agricultural liming to riverine bicarbonate export and CO2 sequestration in the Ohio River basin. Global Biogeochem Cycles 20:GB3012.
Oh NH, Hofmockel M, Lavine ML, Richter DD. 2007. Did elevated atmospheric CO2 alter soil mineral weathering?: an analysis of 5-year soil water chemistry data at Duke FACE study. Glob Change Biol 13:2626–41.
Paustian K, Collins HP, Paul EA. 1997. Management controls on soil carbon. In: Paul EA et al., Eds. Soil organic matter in temperate agro-ecosystems—Long-term experiments in North America. New York: CRC Press. p 15–42.
Pearson M, Davies WJ, Mansfield TA. 1995. Asymmetric responses of adaxial and abaxial stomata to elevated CO2—impacts on the control of gas-exchange by leaves. Plant Cell Environ 18(8):837–43.
Pendall E, Leavitt SW, Brooks T, Kimball BA, Pinter PJ, Wall GW, LaMorte RL, Wechsung G, Wechsung F, Adamsen F, Matthias AD, Thompson TL. 2001. Elevated CO2 stimulates soil respiration in a FACE wheat field. Basic Appl Ecol 2:193–201.
Ren R, Mi F, Bai N. 2000. A chemometrics analysis on the data of precipitation chemistry of China. J Beijing Polytech Univ 26:90–5 (in Chinese with English abstract).
Saxe H, Ellsworth DS, Heath J. 1998. Tansley Review No. 98 Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436.
Schlesinger WH, Lichter J. 2001. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature 411:466–9.
Six J, Conant RT, Paul EA, Paustian K. 2002. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–76.
Smedberg E, Morth CM, Swaney DP, Humborg C. 2006. Modeling hydrology and silicon-carbon interaction in taiga and tundra biomes from a landscape perspective: implications for global warming feedbacks. Global Biogeochem Cycles 20:GB2014. doi:10.1029/2005GB002567.
Suwa M, Katul GG, Oren R, Andrews J, Pippen J, Mace A, Schlesinger WH. 2004. Impact of elevated atmospheric CO2 on forest floor respiration in a temperate pine forest. Global Biogeochem Cycles 18:GB2013. doi:10.1029/2003GB002182.
Tang XL, Liu SG, Zhou GY, Zhang DQ, Zhou CY. 2006. Soil atmospheric exchange of CO2, CH4 and N2O in three subtropical forest ecosystems in southern China. Glob Change Biol 12:546–60.
Vuai SAH, Tokuyama A. 2007. Solute generation and CO2 consumption during silicate weathering under subtropical, humid climate, northern Okinawa Island, Japan. Chem Geol 236:199–216.
Williams EL, Walter LM, Ku TCW, Kling GW, Zak DR. 2003. Effects of CO2 and nutrient availability on mineral weathering in controlled tree growth experiments. Global Biogeochem Cycles. 17(2), GB1041.
Acknowledgments
This study was jointly funded by National Natural Science Foundation of China (Grant No. 31070439), the National Key Technology R&D Program (Grant No. 2009BADC6B02), the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KSCX2-EW-Q-8) and the Natural Science Foundation of Guangdong Province,China (Grant No. 8351065005000001). Meteorological Bureau of Guangdong Province is acknowledged for providing rainfall and temperature data of Guangzhou City from their on-site weather station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
JL conceived the project, designed, and carried out the survey and manipulation, analyzed data, and drafted the manuscript. ZX and DZ contributed to the design of the study, interpretation of results. GZ conceived and designed the study, assisted in writing of paper, supported the research. QD performed the measurement of soil moisture and soil CO2 flux. HD, LZ and CW performed the chemical analysis of soil and soil leachate samples.
Rights and permissions
About this article
Cite this article
Liu, J., Xu, Z., Zhang, D. et al. Effects of Carbon Dioxide Enrichment and Nitrogen Addition on Inorganic Carbon Leaching in Subtropical Model Forest Ecosystems. Ecosystems 14, 683–697 (2011). https://doi.org/10.1007/s10021-011-9438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-011-9438-6