Abstract
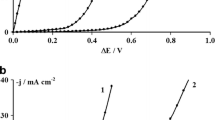
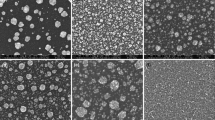
The work presents results of the studies on the synthesis of Co–Pd alloys from acid electrolytes containing chloride ions. The main aim of the tests was to identify reactions responsible for alloy formation and to determine an influence of the electrolysis parameters, i.e. working electrode potential, electrolyte composition and temperature on the composition of the resulted alloy coatings. Electrochemical investigations were performed by applying cyclic voltammetry (CV) combined with electrochemical quartz crystal microbalance (EQCM). The electrolyte composition was selected based on a thermodynamic analysis and spectrophotometric tests which were described in our previous papers [1, 2]. They allowed determination of equilibrium distribution of the metals complex forms and a stability analysis of the electrolyte. The alloys were synthesized within the potential range from −0.7 to −1.1 V. The tests indicate a possibility of alloys synthesis already at the potential range < −0.5 V. The alloys composition was analysed with the use of the EDS technique. The obtained alloys featured Pd content from 0.84 to 71.37 at.%.








Similar content being viewed by others
References
Mech K, Żabiński P, Kowalik R (2013) Co-reduction of electrochemically active [Co(H2O)6]2+ and [CoCl(H2O)5]+ complexes onto gold electrode. J Electrochem Soc 160(6):D246–D250
Mech K, Żabiński P, Kowalik R, Fitzner K (2013) Kinetics and mechanism of [PdClx(H2O)4 − x]2 − x (x = 3,4) complexes electro-reduction. J Electrochem Soc 160(10):H770–H774
Zhang L, Lee KC, Zhang JJ (2007) Effect of synthetic reducing agents on morphology and ORR activity of carbon-supported nano-Pd–Co alloy electrocatalysts. Electrochim Acta 52(28):7964–7971
Savadogo O, Lee K, Oishi K, Mitsushima S, Kamiya N, Ota KI (2004) New palladium alloys catalyst for the oxygen reduction reaction in an acid medium. Electrochem Commun 6(2):105–109
Liu H, Li W, Manthiram A (2009) Factors influencing the electrocatalytic activity of Pd100−xCox (0 < = x < = 50) nanoalloys for oxygen reduction reaction in fuel cells. Appl Catal B-Environ 90(1–2):184–194
Di Noto V, Negro E, Lavina S, Gross S, Pace G (2007) Pd–Co carbon-nitride electrocatalysts for polymer electrolyte fuel cells. Electrochim Acta 53(4):1604–1617
Zuluaga S, Stolbov S (2011) Factors controlling the energetics of the oxygen reduction reaction on the Pd–Co electro-catalysts: insight from first principles. J Chem Phys 135(13):134702
Wang WM, Zheng D, Du C, Zou ZQ, Zhang XG, Xia BJ, Yang H, Akins DL (2007) Carbon-supported Pd–Co bimetallic nanoparticles as electrocatalysts for the oxygen reduction reaction. J Power Sources 167(2):243–249
Suo YG, Zhuang L, Lu JT (2007) First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction. Angew Chem Int Edit 46(16):2862–2864
Takata FM, Sumodjo PTA (2007) Electrodeposition of magnetic CoPd thin films: Influence of plating condition. Electrochim Acta 52(20):6089–6096
Żabiński P, Mech K, Kowalik R (2013) Electrocatalytically active Co–W and Co–W–C alloys electrodeposited in a magnetic field. Electrochim Acta 104:542–548
Gomez E, Ramirez J, Valles E (1998) Electrodeposition of Co–Ni alloys. J Appl Electrochem 28(1):71–79
Rashwan SM (1999) Electrodeposition of Co–Ni alloys from citrate bath onto steel substrate. Metall 53(12):686–691
Żabiński P, Mech K, Kowalik R (2012) Hydrogen evolution on binary and ternary cobalt alloys deposited with superimposed magnetic field. J Iron Steel Res Int 19:1152–1157
Żabiński PR, Mech K, Kowalik R (2012) Co–Mo and Co–Mo–C alloys deposited in a magnetic field of high intensity and their electrocatalytic properties. Arch Met and Mat 57(1):127–133
Sadakov GA, Mazin AA, Gordienko VV, Urin OV, Golovchanskaya RG (1981) Electrodeposition of Co–Fe alloys from sulfamate electrolytes, their structure and properties. Sov Electrochem 17(12):1512–1515
Mentar L, Khelladi MR, Azizi A, Kahoul A (2012) Influence of organic additives on electrodeposition of Co–Cu alloys from sulphate bath. T I Met Finish 90(2):98–104
Zana I, Zangari G (2003) Electrodeposition of Co–Pt films with high perpendicular anisotropy. Electrochem Solid St 6(12):C153–C156
Denbroeder FJA, Donkersloot HC, Draaisma HJG, Dejonge WJM (1987) Magnetic properties and structure of Pd/Co and Pd/Fe multilayers. J Appl Phys 61(8):4317–4319
Hashimoto S, Ochiai Y, Aso K (1989) Perpendicular magnetic-anisotropy in sputtered CoPd alloy films. Jpn J Appl Phys 1 28(9):1596–1599
Childress JR, Duvail JL, Jasmin S, Barthelemy A, Fert A, Schuhl A, Durand O, Galtier P (1994) Perpendicular magnetic-anisotropy in CoxPd1-x alloy films grown by molecular beam epitaxy. J Appl Phys 75(10):6412–6414
Gontarz R, Smardz L, Szymanski B, Juzikis P (1993) Magnetic properties of electrolytic Co–Pd alloy films. J Magn Magn Mater 120(1–3):278–280
Osaka T, Tominaka S, Momma T (2008) Electrodeposited Pd–Co catalyst for direct methanol fuel cell electrodes: preparation and characterization. Electrochim Acta 53(14):4679–4686
Mech K, Zabinski P, Kowalik R, Tokarski T, Fitzner K (2013) Electrodeposition of Co–Pd alloys from ammonia solutions and their catalytic activity for hydrogen evolution reaction. J Appl Electrochem. doi:10.1007/s10800-013-0605-7
Mech K, Żabiński P, Kowalik R, Fitzner K (2013) Analysis of Co–Pd alloys deposition from electrolytes based on [Co(NH3)6]3+ and [Pd(NH3)4]2+ complexes. Electrochim Acta 104:468–473
Mech K, Żabiński P, Kowalik R, Fitzner K (2012) EQCM, SEC and voltammetric study of kinetics and mechanism of hexaamminecobalt(III) electro-reduction onto gold electrode. Electrochim Acta 81:254–259
Mech K, Żabiński P, Kowalik R, Fitzner K (2012) Voltammetric study of electro-reduction of tetraamminepalladium(II) onto gold electrode. J Electroanal Chem 685:15–20
Acknowledgments
This work was supported by Polish National Science Centre under grant 2011/01/N/ST5/05509 and Polish Ministry of Science and Higher Education under grant IP2012/001072.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mech, K., Boczkal, G., Pałka, P. et al. Synthesis of Co–Pd alloys by co-electroreduction of aquachloro-cobalt(II) and palladium(II) complexes. J Solid State Electrochem 18, 3121–3127 (2014). https://doi.org/10.1007/s10008-013-2363-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2363-1