Abstract
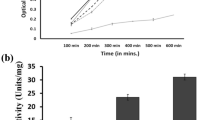
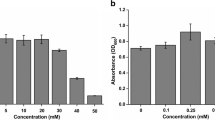
Desulfovibrio alaskensis G20, a sulfate-reducing bacterium, contains an arsRBC2C3 operon that encodes two putative arsenate reductases, DaG20_ArsC2 and DaG20_ArsC3. In this study, resistance assays in E. coli transformed with plasmids containing either of the two recombinant arsenate reductases, showed that only DaG20_ArsC3 is functional and able to confer arsenate resistance. Kinetic studies revealed that this enzyme uses thioredoxin as electron donor and therefore belongs to Staphylococcus aureus plasmid pI258 and Bacillus subtilis thioredoxin-coupled arsenate reductases family. Both enzymes from this family contain a potassium-binding site, but only in Sa_ArsC does potassium actually binds resulting in a lower K m. Important differences between the S. aureus and B. subtilis enzymes and DaG20_ArsC3 are observed. DaG20_ArsC3 contains only two (Asn10, Ser33) of the four (Asn10, Ser33, Thr63, Asp65) conserved amino acid residues that form the potassium-binding site and the kinetics is not significantly affected by the presence of either potassium or sulfate ions. Isothermal titration calorimetry measurements confirmed nonspecific binding of K+ and Na+, corroborating the non-relevance of these cations for catalysis. Furthermore, the low K m and high k cat values determined for DaG20_ArsC3 revealed that this enzyme is the most catalytically efficient potassium-independent arsenate reductase described so far and, for the first time indicates that potassium binding is not essential to have low K m, for Trx-arsenate reductases.




Similar content being viewed by others
Abbreviations
- ArsC:
-
Arsenate reductase
- DTT:
-
Dithiothreitol
- ESI-microTOF:
-
Electrospray ionization micro time of flight
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- LB:
-
Luria Broth
- LMW PTPases:
-
Low molecular mass tyrosine phosphatases
- pNPP:
-
4-Nitrophenyl phosphate disodium salt hexahydrate
- SRB:
-
Sulfate-reducing bacterium
- Trx:
-
Thioredoxin
- TrxR:
-
Thioredoxin reductase
References
Wolfe-Simon F, Blum JS, Kulp TR, Gordon GW, Hoeft SE, Pett-Ridge J, Stolz JF, Webb SM, Weber PK, Davies PCW, Anbar AD, Oremland RS (2010) Science 332:1163–1166
Slaughter DC, Macur RE, Inskeep WP (2012) Microbiol Res 167:151–156
Achour-Rokbani A, Cordi A, Poupin P, Bauda P, Billard P (2010) Appl Environ Microbiol 76:948–955
Tsai SL, Singh S, Chen W (2009) Biotechnology 20:659–667
Branco R, Chung AP, Morais PV (2008) BMC Microbiol 8:95
Bhattacharjee H, Rosen BP (2007) In: Nies DH, Silver S (eds) Molecular microbiology of heavy metals. Springer, Heidelberg, pp 371–406
Silver S, Phung LT (2005) Appl Environ Microbiol 71:599–608
Messens J, Silver S (2006) J Mol Biol 362:1–17
Kaur S, Kamli MR, Ali A (2009) Microbiol 59:288–294
Páez-Espino D, Tamames J, Lorenzo V, Cánovas D (2009) Biometals 22:117–130
Guo X, Li Y, Peng K, Hu Y, Li C, Xia B, Jin C (2005) J Biol Chem 280:39601–39608
Qin J, Fu HL, Ye J, Bencze KZ, Stemmler TL, Rawlings DE, Rosen BP (2007) J Biol Chem 282:34346–34355
Messens J, Martins JC, Brosens E, Belle K, Jacobs DM, Willem R, Wyns L (2002) J Biol Inorg Chem 7:146–156
Oremland RS, Stolz JF (2003) Science 300:939–944
Ordóñez E, Van Belle K, Roos G, De Galan S, Letek M, Gil JA, Wyns L, Mateos LM, Messens J (2009) J Biol Chem 284:15107–15116
Villadangos AF, Van Belle K, Wahni K, Dufe VT, Freitas S, Nur H, De Galan S, Gil JA, Collet JF, Matos LM, Messens J (2011) Mol Microbiol 82:998–1014
Martin P, DeMel S, Shi J, Gladysheva T, Gatti DL, Rosen BP, Edwards BFP (2001) Structure 9:1071–1081
Bennet MS, Guan Z, Laurberg M, Su X (2001) PNAS 98:13577–13582
Li Y, Hu Y, Zhang X, Xu H, Lescop E, Xia B, Jin C (2007) J Bio Chem 282:11078–11083
Zegers I, Martins JC, Willem R, Wyns L, Messens J (2001) Nature 8:843–847
Messens J, Hayburn G, Desmyter A, Laus G, Wyns L (1999) Biochemistry 38:16857–16865
Messens J, Molle IV, Vanhaesebrouck P, Limbourg M, Van Belle K, Wahni K, Martins JC, Loris R, Wyns L (2004) J Mol Biol 339:527–537
Messens J, Martins JC, Zegers I, Van Belle K, Brosens E, Wyns L (2003) J Chromatogr B 790:217–227
Roos G, Loverix S, Brosens E, Van Belle K, Wyns L, Geerlings P, Messens J (2006) ChemBioChem 7:981–989
Roos G, Buts L, Van Belle K, Brosens E, Geerlings P, Loris R, Wyns L, Geerlings P, Messens J (2006) J Mol Biol 360:826–838
Lah N, Lah J, Zegers I, Wyns L, Messens J (2003) J Biol Chem 278:24673–24679
Rodionov DA, Dubchak I, Arkin A, Alm E, Gelfand MS (2004) Genome Biol 5:R90
Carepo M, Baptista JF, Pamplona A, Fauque G, Moura JJG, Reis MAM (2002) Anaerobe 8:325–332
Hansen TA (1994) Antonie Van Leeuwenhoek 66:165–185
Steger JL, Vincent C, Ballard JD, Krumholz LR (2002) Appl Environ Microbiol 68:1932–1937
Sarin R, Sharma YD (2006) Gene 376:107–115
Hemme CL, Wall JD (2004) J Integrative Biology 8:43–55
Li X, Krumholz LR (2009) J Bacteriol 191:4924–4933
Li X, Krumholz LR (2007) J Bacteriol 189:3705–3711
Hauser LJ, Land ML, Brown SD, Larimer F, Keller KL, Rapp-Giles BJ, Price MN, Lin M, Bruce DC, Detter JC, Tapia R, Han CS, Goodwin LA, Cheng J-F, Pitluck S, Copeland A, Lucas S, Nolan M, Lapidus AL, Palumbo AV, Wall JD (2011) J Bacteriol 193:4268–4269
Sambrook J, Russel D (2001) In: Molecular cloning—a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 1508–1526
Pinheiro BA, Proctor MR, Martinez-Fleites C, Prates JAM, Money VA, Davies GJ, Bayer EA, Fontes CMGA, Fierobe HP, Gilbert HJ (2008) J Biol Chem 28:18422–18430
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) In: Walker JM (eds) The proteomics protocols handbook. Human Press, San Diego, pp 571–607
Carepo MSP, Azevedo JSN, Porto JIR, Sousa ASB, Batista JS, Silva ALC, Schneider MPC (2004) Genet Mol Res 3:181–194
Li R, Haile JD, Kennelly PJ (2003) J Bacteriol 185:6780–6789
Zhou J, He Q, Hemme CL, Mukhopadhyay A, Hillesland K, Zhou A, He Z, Van Nostrand JD, Hazen TC, Stahl DA, Wall JD, Arkin AP (2011) Nature 9:452–466
Rosen BP (2002) FEBS Lett 529:86–92
Acknowledgments
We would like to thank Fundação para a Ciência e Tecnologia for the grants PEst-C/EQB/LA0006/2011 and PTDC/BIA-PRO/103980/2008 and fellowship SFRH/BD/62051/2009 to CIPN.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nunes, C.I.P., Brás, J.L.A., Najmudin, S. et al. ArsC3 from Desulfovibrio alaskensis G20, a cation and sulfate-independent highly efficient arsenate reductase. J Biol Inorg Chem 19, 1277–1285 (2014). https://doi.org/10.1007/s00775-014-1184-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-014-1184-8