Abstract
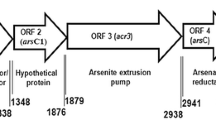
The present study characterized aresenate reductase of Bacillus thuringiensis KPWP1, tolerant to salt, arsenate and a wide range of pH during growth. Interestingly, it was found that arsC, arsB and arsR genes involved in arsenate tolerance are distributed in the genome of strain KPWP1. The inducible arsC gene was cloned, expressed and the purified ArsC protein showed profound enzyme activity with the KM and Kcat values as 25 µM and 0.00119 s−1, respectively. In silico studies revealed that in spite of 19–26% differences in gene sequences, the ArsC proteins of Bacillus thuringiensis, Bacillus subtilis and Bacillus cereus are structurally conserved and ArsC structure of strain KPWP1 is close to nature. Docking and analysis of the binding site showed that arsenate ion interacts with three cysteine residues of ArsC and predicts that the ArsC from B. thuringiensis KPWP1 reduces arsenate by using the triple Cys redox relay mechanism.




Similar content being viewed by others
Availability of data and material
In this study all data generated or analyzed are included in the article.
Code availability
Not applicable.
References
Alfasi H, Friedberg D, Friedberg I (1979) Phosphate transport in arsenate-resistant mutants of Micrococcus lysodeikticus. J Bacteriol 137:69–72. https://doi.org/10.1128/jb.137.1.69-72.1979
Allenby NE, O’Connor N, Prágai Z, Ward AC, Wipat A, Harwood CR (2005) Genome-wide transcriptional analysis of the phosphate starvation stimulon of Bacillus subtilis. J Bacteriol 187:8063–8080. https://doi.org/10.1128/JB.187.23.8063-8080.2005
Altowayti WAH, Algaifi HA, Bakar SA, Shahir S (2019) The adsorptive removal of As (III) using biomass of arsenic resistant Bacillus thuringiensis strain WS3: characteristics and modelling studies. Ecotoxicol Environ Saf 172:176–185. https://doi.org/10.1016/j.ecoenv.2019.01.067
Anderson CR, Cook GM (2004) Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol 48:341–347. https://doi.org/10.1007/s00284-003-4205-3
Antonucci I, Gallo G, Limauro D, Contursi P, Ribeiro AL, Blesa A, Berenguer J, Bartolucci S, Fiorentino G (2017) An ArsR/SmtB family member regulates arsenic resistance genes unusually arranged in Thermus thermophilus HB27. Microbial Biotechnol 10:1690–1701. https://doi.org/10.1111/1751-7915.12761
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. https://doi.org/10.1093/bioinformatics/bti770
Banerjee P, Soni J, Purwar H, Ghosh N, Sengupta TK (2013) Probing the fractal pattern and organization of Bacillus thuringiensis bacteria colonies growing under different conditions using quantitative spectral light scattering polarimetry. J Biomed Opt 18:035003–035003. https://doi.org/10.1117/1.JBO.18.3.035003
Fekih IB, Zhang C, Li YP, Zhao AHA, Saquib Q, Rensing C, Cervantes C (2018) Distribution of arsenic resistance genes in prokaryotes. Front Microbiol 9:2473. https://doi.org/10.3389/fmicb.2018.02473
Bennett MS, Guan Z, Laurberg M, Su X-D (2001) Bacillus subtilis arsenate reductase is structurally and functionally similar to low molecular weight protein tyrosine phosphatases. Proc Natl Acad Sci USA 98:13577–13582. https://doi.org/10.1073/pnas.241397198
Boniolo FS, Rodrigues RC, Prata AMR, Lopez ML, Jacinto T, da Silveira MM, Berbert-Molina MA (2012) Oxygen supply in Bacillus thuringiensis fermentations: bringing new insights on their impact on sporulation and δ-endotoxin production. Appl Microbiol Biotechnol 94:625–636. https://doi.org/10.1007/s00253-011-3746-9
Borin DB, Castrejón-Arroyo K, Cruz-Nolasco A, Peña-Rico M, Sagrillo MR et al (2021) Parasporin A13–2 of Bacillus thuringiensis isolates from the Papaloapan Region (Mexico) induce a cytotoxic effect by late apoptosis against breast cancer cells. Toxins 13(7):476. https://doi.org/10.3390/toxins13070476
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Branco R, Chung A-P, Morais VP (2008) Sequencing and expression of two arsenic resistance operons with different functions in the highly arsenic-resistant strain Ochrobactrum tritici SCII24T. BMC Microbiol 8:95. https://doi.org/10.1186/1471-2180-8-95
Butcher BG, Deane SM, Rawlings DE (2000) The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl Environ Microbiol 66:1826–1833. https://doi.org/10.1128/AEM.66.5.1826-1833.2000
Cai J, Salmon K, DuBow MS (1998) A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology 144:2705–2729. https://doi.org/10.1099/00221287-144-10-2705
Carlin A, Shi W, Dey S, Rosen BP (1995) The ars operon of Escherichia coli confers arsenical and antimonial resistance. J Bacteriol 177:981–986. https://doi.org/10.1128/jb.177.4.981-986.1995
Celniker G, Nimrod G, Ashkenazy H, Glaser F, Martz E, Mayrose I, Pupko T, Ben-Tal N (2013) ConSurf: using evolutionary data to raise testable hypotheses about protein function. Israel J Chem 53:199–206. https://doi.org/10.1002/ijch.201200096
Chauhan D, Srivastava PA, Agnihotri V, Yennamalli RM, Priyadarshini R (2019) Structure and function prediction of arsenate reductase from Deinococcus indicus DR1. J Mol Model 25:15. https://doi.org/10.1007/s00894-018-3885-3
Chien M-F, Ho Y-N, Yang H-E, Narita M, Miyauchi K, Endo G, Huang C-C (2019) Identification of a novel arsenic resistance transposon nested in a mercury resistance transposon of Bacillus sp. MB24. Microorganisms 7:566. https://doi.org/10.3390/microorganisms7110566
Daryanto S, Wang L, Jacinthe P-A (2017) Impacts of no-tillage management on nitrate loss from corn, soybean and wheat cultivation: a meta-analysis. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-12383-7
Diorio C, Cai J, Marmor J, Shinder R, DuBow MS (1995) An Escherichia coli chromosomal ars operon homolog is functional in arsenic detoxification and is conserved in gram-negative bacteria. J Bacteriol 177:2050–2056. https://doi.org/10.1128/jb.177.8.2050-2056.1995
Drewniak L, Sklodowska A (2013) Arsenic-transforming microbes and their role in biomining processes. Environ Sci Poll Res 20:7728–7739. https://doi.org/10.1007/s11356-012-1449-0
Duhovny D, Nussinov R, Wolfson H (2002) Efficient unbound docking of rigid molecules. In: Guigó R, Gusfield D (eds) Algorithms in bioinformatics. Springer, Berlin, pp 185–200. https://doi.org/10.1007/3-540-45784-4_14
Ehrlich HL (1978) Inorganic energy sources for chemolithotrophic and mixotrophic bacteria. Geomicrobiol J 1:65–83. https://doi.org/10.1080/01490457809377724
Firrincieli A, Presentato A, Favoino G, Marabottini R, Allevato E, Stazi SR, Mugnozza GS, Harfouche A, Petruccioli M, Turner RJ, Zannoni D, Cappelletti M (2019) Identification of resistance genes and response to arsenic in Rhodococcus aetherivorans BCP1. Front Microbiol 10:888. https://doi.org/10.3389/fmicb.2019.00888
Gladysheva TB, Oden KL, Rosen BP (1994) Properties of the arsenate reductase of plasmid R773. Biochemistry 33:7288–7293. https://doi.org/10.1021/bi00189a033
Glasser NR, Oyala PH, Osborne TH, Santini JM, Newman DK (2018) Structural and mechanistic analysis of the arsenate respiratory reductase provides insight into environmental arsenic transformations. Proc Nat Acad Sci 115:E8614–E8623. https://doi.org/10.1073/pnas.1807984115
Goddard T et al (2018) UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27:14–25. https://doi.org/10.1002/pro.3235
Gu Y, Nostrand JDV, Wu L, He Z, Qin F-J, Zhou J (2017) Bacterial community and arsenic functional genes diversity in arsenic contaminated soils from different geographic locations. PLoS ONE 12:e0176696. https://doi.org/10.1371/journal.pone.0176696
Hu Y, Nguyen TT, Lee ACY et al (2018) Bacillus thuringiensis Cry5B protein as a new pan-hookworm cure. Int J Parasitol Drugs Drug Resist 8(2):287–294. https://doi.org/10.1016/j.ijpddr.2018.05.001
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16. https://doi.org/10.1016/s0378-4274(02)00084-x
Jain S, Saluja B, Gupta A, Marla SS, Goel R (2011) Validation of arsenic resistance in Bacillus cereus strain AG27 by comparative protein modeling of arsC gene product. Protein J 30:91–101. https://doi.org/10.1007/s10930-011-9305-5
Ji G, Silver S (1992) Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc Nat Acad Sci USA 89(20):9474–9478. https://doi.org/10.1073/pnas.89.20.9474
Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T (2009) The SWISS-MODEL repository and associated resources. Nucl Acids Res 37:D387–D392. https://doi.org/10.1093/nar/gkn750
Kruger MC, Bertin PN, Heipieper HJ, Arsène-Ploetze F (2013) Bacterial metabolism of environmental arsenic—mechanisms and biotechnological applications. Appl Microbiol Biotechnol 97:3827–3841. https://doi.org/10.1007/s00253-013-4838-5
Li X, Krumholz LR (2007) Regulation of arsenate resistance in Desulfovibrio desulfuricans G20 by an arsRBCC operon and an arsC gene. J Bacteriol 189(10):3705–3711. https://doi.org/10.1128/JB.01913-06
Li R, Haile JD, Kennelly PJ (2003) An arsenate reductase from Synechocystis sp. strain PCC 6803 exhibits a novel combination of catalytic characteristics. J Bacteriol 185:6780–6789. https://doi.org/10.1128/JB.185.23.6780-6789.2003
Li Y, Hu Y, Zhang X, Xu H, Lescop E, Xia B, Jin C (2007) Conformational fluctuations coupled to the thiol-disulfide transfer between thioredoxin and arsenate reductase in Bacillus subtilis. J Biol Chem 282:11078–11083. https://doi.org/10.1074/jbc.M700970200
Liu J, Gladysheva TB, Lee L, Rosen BP (1995) Identification of an essential cysteinyl residue in the ArsC arsenate reductase of plasmid R773. Biochemistry 34:13472–13476. https://doi.org/10.1021/bi00041a026
Lloyd JR, Oremland RS (2006) Microbial transformations of arsenic in the environment: from soda lakes to aquifers. Elements (chantilly, VA, US) 2:85–90. https://doi.org/10.2113/gselements.2.2.85
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol. https://doi.org/10.1155/2011/431287
Messens J, Hayburn G, Desmyter A, Laus G, Wyns L (1999) The essential catalytic redox couple in arsenate reductase from Staphylococcus aureus. Biochemistry 38:16857–16865. https://doi.org/10.1021/bi9911841
Nurchi VM, Djordjevic AB, Crisponi G, Alexander J, Bjørklund G, Aaseth J (2020) Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules 10:235. https://doi.org/10.3390/biom10020235
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300:939–944. https://doi.org/10.1126/science.1081903
Pandey S, Shrivastava AK, Singh VK, Rai R, Singh PK, Rai S, Rai LC (2013) A new arsenate reductase involved in arsenic detoxification in Anabaena sp. PCC7120. Funct Integr Genom 13:43–55. https://doi.org/10.1007/s10142-012-0296-x
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Potts AH, Vakulskas CA, Pannuri A, Yakhnin H, Babitzke P, Romeo T (2017) Global role of the bacterial post-transcriptional regulator CsrA revealed by integrated transcriptomics. Nat Commun 8:1–15. https://doi.org/10.1038/s41467-017-01613-1
Qi Y, Kobayashi Y, Hulett FM (1997) The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the pho regulon. J Bacteriol 179:2534–2539. https://doi.org/10.1128/jb.179.8.2534-2539.1997
Rascovan N, Maldonado J, Vazquez MP, Farías ME (2016) Metagenomic study of red biofilms from Diamante Lake reveals ancient arsenic bioenergetics in haloarchaea. ISME J 10:299–309. https://doi.org/10.1038/ismej.2015.109
Rosenberg H, Medveczky N, La Nauze JM (1969) Phosphate transport in Bacillus cereus. Biochimica Et Biophysica Acta Biomembr 193:159–167. https://doi.org/10.1016/0005-2736(69)90069-8
Roy MK, Banerjee P, Sengupta TK, Dattagupta S (2010) Glucose induced fractal colony pattern of Bacillus thuringiensis. J Theor Biol 265:389–395. https://doi.org/10.1016/j.jtbi.2010.05.016
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory
Santo CE, Lam EW, Elowsky CG, Quaranta D, Domaille DW, Chang CJ, Grass G (2011) Bacterial killing by dry metallic copper surfaces. Appl Environ Microbiol 77:794–802. https://doi.org/10.1128/aem.01599-10
Sato T, Kobayashi Y (1998) The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J Bacteriol 180:1655–1661. https://doi.org/10.1128/jb.180.7.1655-1661.1998
Serrato-Gamiño N, Salgado-Lora MG, Chávez-Moctezuma MP, Campos-García J, Cervantes C (2018) Analysis of the ars gene cluster from highly arsenic-resistant Burkholderia xenovorans LB400. World J Microbiol Biotechnol 34:1–10. https://doi.org/10.1007/s11274-018-2526-4
Shen Z, Han J, Wang Y, Sahin O, Zhang Q (2013) The contribution of ArsB to arsenic resistance in Campylobacter jejuni. PLoS ONE 8:e58894. https://doi.org/10.1371/journal.pone.0058894
Shi J, Vlamis-Gardikas A, Åslund F, Holmgren A, Rosen BP (1999) Reactivity of Glutaredoxins 1, 2, and 3 from Escherichia coli shows that Glutaredoxin 2 is the primary hydrogen donor to ArsC-catalyzed arsenate reduction. J Biol Chem 274:36039–36042. https://doi.org/10.1074/jbc.274.51.36039
Silver S, Phung LT (2005) Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl Environ Microbiol 71:599–608. https://doi.org/10.1128/AEM.71.2.599-608.2005
Suhadolnik ML, Salgado APC, Scholte LLS, Bliecher L, Costa PS, Reis MP, Dias MF, Avila MP, Barbosa FAR, Chartone-SouzaE NAMA (2017) Novel arsenic-transforming bacteria and the diversity of their arsenic-related genes and enzymes arising from arsenic-polluted freshwater sediment. Sci Rep 7:1–17. https://doi.org/10.1038/s41598-017-11548-8
Vargas-Blanco DA, Shell SS (2020) Regulation of mRNA stability during bacterial stress responses. Front Microbiol. https://doi.org/10.3389/fmicb.2020.02111
Wang Y, Fu J, Zhu Q, Zhu L, Zheng J, Liu H, Peng D, Ruan L, Sun M (2016) Complete genome sequence of Bacillus thuringiensis serovar alesti BGSC 4C1, a typical strain with toxicity to Lepidoptera insects. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2016.09.015
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucl Acids Res 35:W407–W410. https://doi.org/10.1093/nar/gkm290
Wu J, Rosen BP (1993) The arsD gene encodes a second trans-acting regulatory protein of the plasmid-encoded arsenical resistance operon. Mol Microbiol 8:615–623. https://doi.org/10.1111/j.1365-2958.1993.tb01605.x
Yan G, Chen X, Du S, Deng Z, Wang L, Chen S (2019) Genetic mechanisms of arsenic detoxification and metabolism in bacteria. Curr Genet 65:329–338. https://doi.org/10.1007/s00294-018-0894-9
Acknowledgements
The authors acknowledge the service of SGS laboratory, Kolkata for atomic absorption spectroscopy facility. We also acknowledge the service of 1st BASE for DNA sequencing. Authors acknowledge Arpita Karmakar for technical assistance. Authors also like to thank Dr. Sumita Sengupta (Calcutta University) for her valuable suggestions regarding the manuscript writing. PB was a recipient of a Senior Research Fellowship from the Council of Scientific and Industrial Research, Government of India. AC, NB and SJ were recipients of Research Fellowship from Indian Institute of Science Education and Research (IISER) Kolkata. The work was supported by IISER Kolkata.
Funding
The study was funded by IISER Kolkata.
Author information
Authors and Affiliations
Contributions
PB, AC, PPD and TKS conceived the study and designed the experiments. PB, AC, NB and SJ performed the experiments and analysed the data. PB and TKS have written the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of the paper declare that there is no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banerjee, P., Chatterjee, A., Jha, S. et al. Biochemical, molecular and in silico characterization of arsenate reductase from Bacillus thuringiensis KPWP1 tolerant to salt, arsenic and a wide range of pH. Arch Microbiol 204, 46 (2022). https://doi.org/10.1007/s00203-021-02660-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02660-5