Abstract
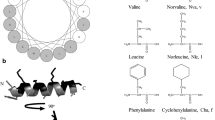
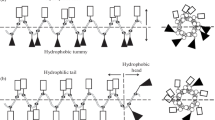
Design of antimicrobial peptides with selective activity towards microorganisms is an important step towards the development of new antimicrobial agents. Leucine zipper sequence has been implicated in cytotoxic activity of naturally occurring antimicrobial peptides; moreover, this motif has been utilized for the design of novel antimicrobial peptides with modulated cytotoxicity. To understand further the impact of substitution of amino acids at ‘a’ and/or ‘d’ position of a leucine zipper sequence of an antimicrobial peptides on its antimicrobial and cytotoxic properties four short peptides (14-residue) were designed on the basis of a leucine zipper sequence without or with replacement of leucine residues in its ‘a’ and ‘d’ positions with d-leucine or alanine or proline residue. The original short leucine zipper peptide (SLZP) and its d-leucine substituted analog, DLSA showed comparable activity against the tested Gram-positive and negative bacteria and the fungal strains. The alanine substituted analog (ASA) though showed appreciable activity against the tested bacteria, it showed to some extent lower activity against the tested fungi. However, the proline substituted analog (PSA) showed lower activity against the tested bacterial or fungal strains. Interestingly, DLSA, ASA and PSA showed significantly lower cytotoxicity than SLZP against both human red blood cells (hRBCs) and murine 3T3 cells. Cytotoxic and bactericidal properties of these peptides matched with peptide-induced damage/permeabilization of mammalian cells and bacteria or their mimetic lipid vesicles suggesting cell membrane could be the target of these peptides. As evidenced by tryptophan fluorescence and acrylamide quenching studies the peptides showed similarities either in interaction or in their localization within the bacterial membrane mimetic negatively charged lipid vesicles. Only SLZP showed localization inside the mammalian membrane mimetic zwitterionic lipid vesicles. The results show significant scope for designing antimicrobial agents with selectivity towards microorganisms by substituting leucine residues at ‘a’ and/or ‘d’ positions of a leucine zipper sequence of an antimicrobial peptide with different amino acids.






Similar content being viewed by others
Abbreviations
- PBS:
-
Phosphate buffered saline
- FITC:
-
Fluorescin isothiocyanate
- Fmoc:
-
N-(9-fluorenyl) methoxycarbonyl
- DIC:
-
Differential interference contrast
- HPLC:
-
High-performance liquid chromatography
- ESI-MS:
-
Electron spray ionization- mass spectrometry
- ANS:
-
8-Anilinonapthalene-1-sulfonic acid
- DiS-C3-5:
-
3,3′-Dipropylthiadicarbocyanine iodide
References
Ahmad A, Yadav SP et al (2006) Utilization of an amphipathic leucine zipper sequence to design antibacterial peptides with simultaneous modulation of toxic activity against human red blood cells. J Biol Chem 281(31):22029–22038
Ahmad A, Asthana N et al (2009a) Structure-function study of cathelicidin-derived bovine antimicrobial peptide BMAP-28: design of its cell-selective analogs by amino acid substitutions in the heptad repeat sequences. Biochim Biophys Acta 1788(11):2411–2420
Ahmad A, Azmi S et al (2009b) Design of nontoxic analogues of cathelicidin-derived bovine antimicrobial peptide BMAP-27: the role of leucine as well as phenylalanine zipper sequences in determining its toxicity. Biochemistry 48(46):10905–10917
Allen TM, Cleland LG (1980) Serum-induced leakage of liposome contents. Biochim Biophys Acta 597(2):418–426
Asthana N, Yadav SP et al (2004) Dissection of antibacterial and toxic activity of melittin: a leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J Biol Chem 279(53):55042–55050
Azmi S, Srivastava S et al (2013) Characterization of antimicrobial, cytotoxic, and antiendotoxin properties of short peptides with different hydrophobic amino acids at “a” and “d” positions of a heptad repeat sequence. J Med Chem 56(3):924–939
Bhunia A, Mohanram H et al (2009) Lipopolysaccharide bound structures of the active fragments of fowlicidin-1, a cathelicidin family of antimicrobial and antiendotoxic peptide from chicken, determined by transferred nuclear Overhauser effect spectroscopy. Biopolymers 92(1):9–22
Bolognesi B, Kumita JR et al (2010) ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol 5(8):735–740
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Brogden NK, Brogden KA (2011) Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int J Antimicrob Agents 38(3):217–225
Castellano FN, Lakowicz JR (1998) A water-soluble luminescence oxygen sensor. Photochem Photobiol 67(2):179–183
Chen Y, Guarnieri MT et al (2007) Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother 51(4):1398–1406
Crisma M, Fasman GD et al (1984) Peptide models for beta-turns. A circular dichroism study. Int J Pept Protein Res 23(4):411–419
Cudic M, Otvos L Jr (2002) Intracellular targets of antibacterial peptides. Curr Drug Targets 3(2):101–106
Deslouches B, Islam K et al (2005) Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob Agents Chemother 49(8):3208–3216
Ghosh JK, Ovadia M et al (1997) A leucine zipper motif in the ectodomain of Sendai virus fusion protein assembles in solution and in membranes and specifically binds biologically-active peptides and the virus. Biochemistry 36(49):15451–15462
Giuliani A, Rinaldi AC (2011) Beyond natural antimicrobial peptides: multimeric peptides and other peptidomimetic approaches. Cell Mol Life Sci 68(13):2255–2266
Greenfield N, Fasman GD (1969) Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8(10):4108–4116
Gustiananda M, Liggins JR et al (2004) Conformation of prion protein repeat peptides probed by FRET measurements and molecular dynamics simulations. Biophys J 86(4):2467–2483
Haldar S, Raghuraman H et al (2010) Membrane interaction of the N-terminal domain of chemokine receptor CXCR1. Biochim Biophys Acta 1798(6):1056–1061
Hultmark D (2003) Drosophila immunity: paths and patterns. Curr Opin Immunol 15(1):12–19
Javadpour MM, Barkley MD (1997) Self-assembly of designed antimicrobial peptides in solution and micelles. Biochemistry 36(31):9540–9549
Jiang Z, Kullberg BJ et al (2008) Effects of hydrophobicity on the antifungal activity of alpha-helical antimicrobial peptides. Chem Biol Drug Des 72(6):483–495
Loew LM, Rosenberg I et al (1983) Diffusion potential cascade. Convenient detection of transferable membrane pores. Biochemistry 22(4):837–844
Ma QQ, Lv YF et al (2013) Rational design of cationic antimicrobial peptides by the tandem of leucine-rich repeat. Amino Acids 44(4):1215–1224
Matulis D, Baumann CG et al (1999) 1-Anilino-8-naphthalene sulfonate as a protein conformational tightening agent. Biopolymers 49(6):451–458
Nicolas P (2009) Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J 276(22):6483–6496
Pandey BK, Ahmad A et al (2010) Cell-selective lysis by novel analogues of melittin against human red blood cells and Escherichia coli. Biochemistry 49(36):7920–7929
Pandey BK, Srivastava S et al (2011) Inducing toxicity by introducing a leucine-zipper-like motif in frog antimicrobial peptide, magainin 2. Biochem J 436(3):609–620
Papo N, Braunstein A et al (2004) Suppression of human prostate tumor growth in mice by a cytolytic D-, L-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res 64(16):5779–5786
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462(1–2):55–70
Sims PJ, Waggoner AS et al (1974) Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 13(16):3315–3330
Subbalakshmi C, Nagaraj R et al (1999) Biological activities of C-terminal 15-residue synthetic fragment of melittin: design of an analog with improved antibacterial activity. FEBS Lett 448(1):62–66
Suh JY, Lee YT et al (1999) Structural and functional implications of a proline residue in the antimicrobial peptide gaegurin. Eur J Biochem 266(2):665–674
Varkey J, Nagaraj R (2005) Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines. Antimicrob Agents Chemother 49(11):4561–4566
Weber G, Shinitzky M (1970) Failure of energy transfer between identical aromatic molecules on excitation at the long wave edge of the absorption spectrum. Proc Natl Acad Sci USA 65(4):823–830
Wu CS, Ikeda K et al (1981) Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry 20(3):566–570
Yadav SP, Kundu B et al (2003) Identification and characterization of an amphipathic leucine zipper-like motif in Escherichia coli toxin hemolysin E. Plausible role in the assembly and membrane destabilization. J Biol Chem 278(51):51023–51034
Yadav SP, Ahmad A et al (2008) Inhibition of lytic activity of Escherichia coli toxin hemolysin E against human red blood cells by a leucine zipper peptide and understanding the underlying mechanism. Biochemistry 47(7):2134–2142
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395
Zhu WL, Nan YH et al (2007a) Cell selectivity of an antimicrobial peptide melittin diastereomer with D-amino acid in the leucine zipper sequence. J Biochem Mol Biol 40(6):1090–1094
Zhu WL, Song YM et al (2007b) Substitution of the leucine zipper sequence in melittin with peptoid residues affects self-association, cell selectivity, and mode of action. Biochim Biophys Acta 1768(6):1506–1517
Acknowledgments
CSIR-CDRI communication number of this manuscript is 8728. We are extremely grateful to Dr. S. K. Puri, Parasitology Division, CSIR-CDRI for kindly providing us fresh hRBCs for performing hemolytic activity assays with our peptides. The work was supported by the CSIR network projects NWP 0005 and BioDiscovery (BSC 0120). AA, SA, SS acknowledges the receipt of senior research fellowships from Council of Scientific and Industrial Research (CSIR), India; AK and JKT receipt of senior research fellowships from ICMR and UGC. The authors are extremely thankful to AL Vishwakarma for recoding the flow cytometry profiles and Sanjeev Kanojiya, Sophisticated Analytical Instrumentation Facility (SAIF), CSIR-CDRI for recording the ES-MS mass spectra.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Ahmad and S. Azmi contributed equally to the work. S. Srivastava and A. Kumar contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmad, A., Azmi, S., Srivastava, S. et al. Design and characterization of short antimicrobial peptides using leucine zipper templates with selectivity towards microorganisms. Amino Acids 46, 2531–2543 (2014). https://doi.org/10.1007/s00726-014-1802-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1802-3