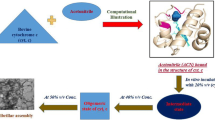
Abstract
Aggregation of protein into insoluble intracellular complexes and inclusion bodies underlies the pathogenesis of human neurodegenerative diseases. Importance of cytochrome c (cyt c) arises from its involvement in apoptosis, sequence homology and for studying molecular evolution. A systemic investigation of polyethylene glycol (PEG) and trifluoroethanol (TFE) on the conformational stability of cyt c as a model hemeprotein was made using multi-methodological approach. Cyt c exists as molten globule (MG) at 60 % PEG-400 and 40 % TFE as confirmed by far-UV CD, attenuated total reflection Fourier transform infrared spectroscopy, Trp environment, 8-anilino-1-naphthalene-sulfonic acid (ANS) binding and blue shift in the soret band. Q-band splitting in MG states specifies conformational changes in the hydrophobic heme-binding pocket. Aggregates were detected at 90 % PEG-400 and 50 % TFE as confirmed by increase thioflavin T and ANS fluorescence and shift in Congo red absorbance. Detection of prefibrils and protofibrils at 90 % PEG-400 and 50 % TFE was possible after 72-h incubation. Single cell gel electrophoresis of prefibrils and protofibrils showed DNA damage confirming their toxicity and potential health hazards. Scanning electron microscopy and XRD analysis confirmed prefibrillar oligomers and protofibrils of cyt c.












Similar content being viewed by others
References
Amani S, Naeem A (2011) Acetonitrile can promote formation of different structural intermediate states on aggregation pathway of immunoglobulin G from human and bovine. Int J Biol Macromol 49:71–78
Amani S, Naeem A (2013a) Understanding protein folding from globular to amyloid state. Aggregation: darker side of protein. Process Biochem 48:1651–1664
Amani S, Naeem A (2013b) Detection and analysis of amorphous aggregates and fibrils of cytochrome c in the presence of phenolic acid. Int J Biol Macromol 58:104–112
Arunkumar AI, Kumar TKS, Yu C (1997a) Non-specific helix-induction in charged homopolypeptides by alcohols. Biochim Biophys Acta 1338:69–76
Arunkumar AI, Kumar TKS, Yu C (1997b) Specificity of helix-induction by 2,2,2-trifluoroethanol in polypeptides. Int J Biol Macromol 21:223–230
Blauer G, Sreerama N, Woody RW (1993) Optical activity of hemoproteins in the Soret region. Circular dichroism of heme undecapeptide of cytochrome c in aqueous solution. Biochemistry 32:6674–6679
Cammers-Goodwin A, Allen TJ, Oslick SL, McClure KF, Lee JH, Kemp DS (1996) Mechanism of stabilization of helical conformations of polypeptide by water containing trifluoroethanol. J Am Chem Soc 118:3082–3090
Caramelo JJ, Castro OA, Alonso LG, De Prat-Gay G, Parodi AJ (2003) UDP-Glc: glycoprotein glucosyltransferase recognizes structured and solvent accessible hydrophobic patches in molten globule-like folding intermediates. Proc Natl Acad Sci USA 100:86–91
Davies AM, Guillemette JG, Smith M, Greenwood C, Thurgood AGP, Mauk AG, Moore GR (1993) Redesign of the interior hydrophobic region of mitochondrial cytochrome c by site directed mutagenesis. Biochemistry 32:5431–5435
Dong A, Matsuura J, Manning MC, Carpenter JF (1998) Intermolecular β-sheet results from trifluoroethanol- induced nonnative α-helical structure in β-sheet predominant proteins: infrared and circular dichroism spectroscopic study. Arch Biochem Biophys 355:275–281
Dzwolak W, Pecul M (2005) Chiral bias of amyloid fibrils revealed by the twisted conformation of thioflavin T: an induced circular dichroism/DFT study. FEBS Lett 579:6601–6603
Eftink MR, Ghiron CA (1982) Fluorescence quenching studies with proteins. Anal Biochem 114:199–227
Eisert R, Felau L, Brown LR (2006) Methods for enhancing the accuracy and reproducibility of Congo red and thioflavin T assays. Anal Biochem 353:144–146
Fiskum G, Starkov A, Polster BM, Chinopoulos C (2003) Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann NY Acad Sci 991:111–119
Gong J, Yao P, Duan H, Jiang M, Gu S, Chunyu L (2003) Structural transformation of cytochrome c and apo cytochrome c induced by sulfonated polystyrene. Biomacromolecules 4:1293–1300
Herold DA, Rodeheaver GT, Bellamy WT, Fitton LA, Bruns DE, Edlich RF (1982) Toxicity of topical polyethylene glycol. Toxicol Appl Parmacol 6:329–335
Iram A, Naeem A (2013) Detection and analysis of protofibrils and fibrils of hemoglobin: implications for the pathogenesis and cure of heme loss related maladies. Arch Biochem Biophys 533:69–78
Jayakumar R, Murali J, Koteeswari D, Gomathi K (2004) Cytotoxic and membrane perturbation effects of a novel amyloid forming model peptide poly (Leucine-Glutamic Acid). J Biochem 136:457–462
Khan TA, Amani S, Naeem A (2011) Glycation promotes the formation of genotoxic aggregates in glucose oxidase. Amino Acids 43:1311–1322
Khare N, Eggleston CM, Lovelace DM, Boese SW (2006) Structural and redox properties of mitochondrial cytochrome c co-sorbed with phosphate on hematite (α-Fe2O3) surfaces. J Colloid Interface Sci 303:404–414
Kraus A, Groveman BR, Caughey B (2013) Prions and the potential transmissibility of protein misfolding diseases. Annu Rev Microbiol 67:543–564
Margoliash E (1963) Primary structure and evolution of cytochrome c. Proc Natl Acad Sci USA 50:672–679
Mendes SB, Li L, Burke JJ (1996) Broad-band attenuated total reflection spectroscopy of a hydrated protein film on a single mode planar waveguide. Langmuir 12:3374–3376
Myer YP (1968) Conformations of cytochromes. II. Comparative study of circular dichroism spectra, optical rotator dispersion, and absorption spectra of horse heart cytochrome c. J Biol Chem 243:2115–2122
Naeem A, Amani S (2013) Deciphering structural intermediates and genotoxic fibrillar aggregates of albumins: a molecular mechanism underlying for degenerative diseases. PLoS ONE 8:e54061
Naeem A, Fazili NA (2011) Defective protein folding and aggregation as the basis of neurodegenerative diseases: the darker aspect of proteins. Cell Biochem Biophys 61:237–250
Naeem A, Khan RH (2004) Characterization of molten globule state of cytochrome c at alkaline, native and acidic pH induced by butanol and SDS. Int J Biochem Cell Biol 36:2281–2292
Naeem A, Khan TA, Muzaffar M, Ahmad S, Saleemuddin M (2011) A partially folded state of ovalbumin at low pH tends to aggregate. Cell Biochem Biophys 17:271–279
Nelson JW, Kallenbach NR (1986) Stabilization of ribonuclease S peptide by trifluoroethanol. Proteins 1:211–217
Sadqi M, Hernandez F, Pan UM, Perez M, Schaeberle MD, Vila JAA, Munoz V (2002) α-Helix structure in Alzheimer’s disease aggregates of tau-protein. Biochemistry 41:7150–7155
Stryer L (1965) The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol 13:482–495
Stryer L (1968) Fluorescence spectroscopy of protein. Science 162:526–533
Turnell WG, Finch JT (1992) Binding of the dye Congo red to the amyloid protein pig insulin reveals a novel homology amongst amyloid-forming peptide sequences. J Mol Biol 227:1205–1223
Urry DW (1967) The heme chromophore in the ultraviolet. J Biol Chem 242:4441–4448
Wiederkehr WRS, Hoops GC, Aslan MM, Byard CL, Mendes SB (2009) Investigations on the Q and CT bands of cytochrome c submonolayer adsorbed on an alumina surface using broadband spectroscopy with single-mode integrated optical waveguides. J Phys Chem C Nanomater Interfaces 113:8306–8312
Wilson MT, Greenwood C (1996) Cytochrome-c: a multidisciplinary approach. University Science Books, Sausalito
Wood JD, Beaujeux TP, Shaw PJ (2003) Protein aggregation in motor neuron disorder. Neuropathol Appl Neurobiol 29:529–545
Zerovnik E, Skarabot M, Skerget K, Giannini S, Stoka V, Jenko-Kokalj S, Staniforth RA (2007) Amyloid fibril formation by human stefin B: influence of pH and TFE on fibril growth and morphology. Amyloid 14:237–247
Acknowledgments
The authors are highly thankful for the facilities available at AMU Aligarh. Authors are thankful to Indo–US Science and Technology Forum for financial support. USIF, AMU has been highly acknowledged for providing SEM facility. S. A. is the recipient of UGC-SRF.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amani, S., Naeem, A. Deciphering aggregates, prefibrillar oligomers and protofibrils of cytochrome c. Amino Acids 46, 1839–1851 (2014). https://doi.org/10.1007/s00726-014-1698-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1698-y