Abstract
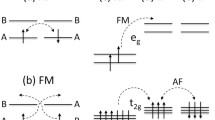
The effect of antisite disorder in the layered sodium iron antimonate Na4FeSbO6 was examined both experimentally and theoretically. The magnetic susceptibility and specific heat measurements show that Na4FeSbO6 does not undergo a long-range antiferromagnetic order, unlike its structural analog Li4FeSbO6. The electron spin resonance yields the complicated picture of coexistence of two magnetic subsystems corresponding to two different Fe cation positions in the lattice (regular and antisite) and driving the magnetic properties of the Na4FeSbO6. This conclusion found perfect confirmation from both the Mössbauer and X-ray absorption data which show the presence of two kinds of Fe3+ ions being in high-spin Fe3+ (S = 5/2) and low-spin Fe3+ (S = 1/2) states. These findings arise from the antisite disorder between the Fe3+ and Sb5+ ions in the (NaFeSbO6)3− layers of Na4FeSbO6. Our density functional calculations show that the Fe3+ ions located at the Sb5+ sites exist as low-spin Fe3+ ions, and that the spins of each Fe3+ (S = 5/2)–Fe3+ (S = 1/2)–Fe3+ (S = 5/2) trimer generated by the antisite disorder has a ferrimagnetic arrangement Fe3+↑–Fe3+↓–Fe3+↑, which enhances the magnetization of Na4FeSbO6 and leads to an apparently positive Curie–Weiss temperature.












Similar content being viewed by others
References
K. Binder, A.P. Young, Rev. Mod. Phys. 58, 801–976 (1986)
A.P. Ramirez, Annu. Rev. Mater. Sci. 24, 453–480 (1994)
J.E. Greedan, J. Mater. Chem. 11, 37–53 (2001)
F.J. Berry, A. Labarta, X. Obradors, R. Rodriguez, M.I. Sarson, J. Tejada, Hyperfine Interact. 41, 463–466 (1988)
A. Labarta, R. Rodriguez, L. Balcells, J. Tejada, X. Obradors, F.J. Berry, Phys. Rev. B 44, 691–698 (1991)
M.T. Causa, M. Tovar, X. Obradors, A. Labarta, J. Tejada, Phys. Rev. B 44, 4455–4460 (1991)
X. Wang, D.A. Vander Griend, C.L. Stern, K.R. Poeppelmeier, J. Alloys Comp. 298, 119–124 (2000)
V. Likodimos, N. Guskos, S. Glenis, R. Szymczak, A. Bezkrovnyi, M. Wabia, J. Typek, G. Gasiorek, M. Kurzawa, I. Rychlowska-Himmel, A. Blonska-Tabero, Eur. Phys. J. B 38, 13–18 (2004)
N. Guskos, V. Likodimos, S. Glenis, G. Zolnierkiewicz, J. Typec, R. Szymczak, A. Blonska-Tabero, J. Appl. Phys. 101, 103922 (2007)
R. Rodriguez, A. Fernandez, A. Isalgue, J. Rodriguez, A. Labarta, J. Tejada, X.J. Obradors, Phys. C 18, L401–L405 (1985)
P.D. Battle, T.C. Gibb, A.J. Herod, S.-H. Kim, P.H. Munns, J. Mater. Chem. 5, 865–870 (1995)
A.P. Ramirez, B. Hessen, M. Winklemann, Phys. Rev. Lett. 84, 2957–2960 (2000)
X. Obradors, A. Labarta, A. Isalgue, J. Tejada, J. Rodriguez, M. Pernet, Solid State Commun. 65, 189–192 (1988)
N.P. Raju, E. Gmelin, R.K. Kremer, Phys. Rev. B 46, 5405–5411 (1992)
A.S. Wills, A.J. Harrison, Chem. Soc. Faraday Trans. 92, 2161–2165 (1996)
A.S. Wills, A. Harrison, C. Ritter, R.I. Smith, Phys. Rev. B 61, 6156–6169 (2000)
K. Hirota, Y. Nakazawa, M. Ishikawa, J. Phys. Cond. Matter. 3, 4721–4730 (1991)
M. Bieringer, J.E. Greedan, G.M. Luke, Phys. Rev. B. 62, 6521–6529 (2000)
P.-H.T. Nguyen, F. Ramezanipour, J.E. Greedan, L.M.D. Cranswick, S. Derakhshan, Inorg. Chem. 51, 11493–11499 (2012)
A.F. Wells, Structural Inorganic Chemistry (Clarendon Press, Oxford, 1986)
A.R. West, Solid State Chemistry and Its Applications (John Wiley & Sons, Chichester-New York-Brisbane-Toronto-Singapore, 1984)
L. Minervini, R.W. Grimes, K.E.J. Sickafus, Amer. Ceram. Soc. 83, 1873–1878 (2000)
G.D. Blundred, C.A. Bridges, M.J. Rosseinsky, Angew. Chem. Int. Ed. 43, 3562–3565 (2004)
M.S. Islam, R. Dominko, C. Masquelier, C. Sirisopanaporn, A.R. Armstrong, P.G. Bruce, J. Mater. Chem. 21, 9811–9818 (2011)
C.A.J. Fisher, N. Kuganathan, M.S.J. Islam, Mater. Chem. A 1, 4207–4214 (2013)
X. Guo, M. Wang, X. Huang, P. Zhao, X. Liu, R.J. Che, Mater. Chem. A 1, 8775–8781 (2013)
V.V. Politaev, V.B. Nalbandyan, Sol. State Sci. 11, 144–150 (2009)
E.A. Zvereva, O.A. Savelieva, Y.D. Titov, M.A. Evstigneeva, V.B. Nalbandyan, C.N. Kao, J.-Y. Lin, I.A. Presniakov, A.V. Sobolev, S.A. Ibragimov, M. Abdel-Hafiez, Y. Krupskaya, C. Jähne, G. Tan, R. Klingeler, B. Büchner, A.N. Vasiliev, Dalton Trans 42, 1550–1566 (2013)
M.-H. Whangbo, H.-J. Koo, D. Dai, J. Solid State Chem. 176, 417–481 (2003)
H.J. Xiang, C. Lee, H.-J. Koo, X.G. Gong, M.-H. Whangbo, Dalton Trans. 42, 823–853 (2013)
M.E. Matsnev, V.S. Rusakov, AIP Conf. Proc. 1489, 178–179 (2012)
Y.-J. Chen, M.G. Jiang, C.W. Luo, J.-Y. Lin, K.H. Wu, J.M. Lee, J.M. Chen, Y.K. Kuo, J.Y. Juang, C.Y. Mou, Phys. Rev. B 88, 134525 (2013)
R.D. Shannon, Acta Crystallogr., Sect. A 32, 751–767 (1976)
W. Schmidt, R. Berthelot, L. Etienne, A. Wattiaux, M.A. Subramanian, Mater. Res Bull. 50, 292–296 (2014)
J.B. Goodenough, Magnetism and the Chemical Bond (Robert E. Krieger Publishing Company, Huntington, NY, 1976)
G.A. Bain, J.F. Berry, J. Chem. Educ. 85, 532–536 (2008)
V.B. Nalbandyan, M. Avdeev, M.A. Evstigneeva, J. Solid State Chem. 199, 62–65 (2013)
A. Tari, The Specific Heat of Matter at Low Temperature (Imperial College Press, London, 2003)
V.A. Ivanshin, J. Deisenhofer, H.-A. Krug von Nidda, A. Loidl, A. Mukhin, J. Balbashov, M.V. Eremin, Phys. Rev. B 61, 6213–6219 (2000)
A. Abragam, B. Bleaney, Electron Paramagnetic Resonance of Transition Ions (Clarendon Press, Oxford, 1970)
W. Low, Paramagnetic Resonance of Solids (Academic Press Inc., New York, 1960)
C.R. Kurkjian, E.A. Sigety, Phys. Chem. Glasses 9, 73 (1968)
D. Loveridge, S. Parke, Phys. Chem. Glasses 12, 90 (1971)
E. Dormann, V. Jaccarino, Phys. Lett. 48A, 81 (1974)
S.B. Oseroff, Phys. Rev. B 25, 6584 (1982)
S. Viticoli, D. Fiorani, M. Nogués, J.L. Dormann, Phys. Rev. B 26, 6085 (1982)
E.A. Zvereva, O.A. Savelieva, A.E. Primenko, S.A. Ibragimov, E.I. Slyn’ko, V.E. Slyn’ko, J. Appl. Phys. 108, 093923 (2010)
D.L. Huber, Phys. Rev. B 6, 3180 (1972)
K. Kawasaki, Prog. Theor. Phys. 39, 285–311 (1968)
K. Kawasaki, Phys. Lett. 26A, 543 (1968)
P.M. Richards, Solid State Commun. 13, 253–256 (1973)
A.G. Anders, S.V. Volotski, J. Magn. Magn. Mater. 31–34, 1169–1170 (1983)
E.A. Zvereva, M.A. Evstigneeva, V.B. Nalbandyan, O.A. Savelieva, S.A. Ibragimov, O.S. Volkova, L.I. Medvedeva, A.N. Vasiliev, R. Klingeler, B. Büchner, Dalton Trans. 41, 572–580 (2012)
E.A. Zvereva, V.B. Nalbandyan, M.A. Evstigneeva, H.-J. Koo, M.-H. Whangbo, A.V. Ushakov, B.S. Medvedev, L.I. Medvedeva, N.A. Gridina, G.E. Yalovega, A.V. Churikov, A.N. Vasiliev, B. Büchner, J. Solid State Chemistry 225, 89–96 (2015)
F. Menil, J. Phys. Chem. Solids 46, 763–789 (1985)
P. Gütlich, E. Bill, A.X. Trautwein, Mössbauer Spectroscopy and Transition Metal Chemistry (Fundamentals and Applications) (Springer, Berlin, 2012)
D.H. Kim, H.J. Lee, G. Kim, Y.S. Koo, J.H. Jung, H.J. Shin, J.-Y. Kim, J.-S. Kang, Phys. Rev. B 79, 033402 (2009)
E. Stavitski, F.M. de Groot, Micron 41, 687 (2010)
J.P. Crocombette, M. Pollak, F. Jollet, N. Thromat, M. Gautier-Soyer, Phys. Rev. B 52, 3143 (1995)
G. Kresse, J. Hafner, Phys. Rev. B 47, 558–561 (1993)
G. Kresse, J. Furthmüller, Comput. Mater. Sci. 6, 15–50 (1996)
G. Kresse, J. Furthmüller, Phys. Rev. B 54, 11169–11186 (1996)
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 77, 3865–3868 (1996)
S.L. Dudarev, G.A. Botton, S.Y. Savrasov, C.J. Humphreys, A.P. Sutton, Phys. Rev. B 57, 1505–1509 (1998)
D. Dai, M.-H. Whangbo, J. Chem. Phys. 114, 2887–2893 (2001)
D. Dai, M.-H. Whangbo, J. Chem. Phys. 118, 29–39 (2003)
Acknowledgments
E.A.Z. and V.B.N. appreciate support from the Russian Foundation for Basic Research (grants 14-02-00245, 11-03-01101). J.-Y.L. acknowledges support from MOE-ATU and National Science Council of Taiwan under Grant No. NSC98-2112-009-005-MY3. The work at NCSU was supported by the computing resources of the NERSC Center and the HPC Center of NCSU.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Figs. 13, 14, 15, 16, 17 and Tables 4, 5, 6, 7.
Unit cell of the (NaFeSbO6)3− layer containing the antisite defects between Fe3+ and Sb5+ ions. The blue circles represent the Fe3+ ions, the cyan circles the Sb5+ ions, the yellow circles the Na atoms, and white circles the O atoms. The oxygen atoms numbered 1–20 are used to specify the Fe–O bond lengths and their magnetic moments. Note that the Fe(1) atom is located at an Sb5+ ion site (color figure online)
Rights and permissions
About this article
Cite this article
Zvereva, E.A., Presniakov, I.A., Whangbo, MH. et al. Crucial Role of Site Disorder and Frustration in Unusual Magnetic Properties of Quasi-2D Triangular Lattice Antimonate Na4FeSbO6 . Appl Magn Reson 46, 1121–1145 (2015). https://doi.org/10.1007/s00723-015-0700-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-015-0700-5