Abstract
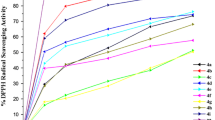
A convenient and environmentally benign procedure for the synthesis of a new series of trifluoromethyl-substituted N-(pyrimidin-2-yl)benzo[d]thiazol-2-amines by the cyclocondensation reaction of (benzo[d]thiazolyl)guanidine with either 4-alkoxy-4-alkyl(aryl/heteroaryl)-1,1,1-trifluoroalk-3-en-2-ones or 2,2,2-trifluoro-1-(2-methoxycyclohexen-1-en-1-yl)ethanone is described. The main reactions were performed in refluxing water as the solvent (8–24 h), without catalysts, and the corresponding new N-(pyrimidinyl)-1H-(benzo[d]thiazolyl)amines were obtained at a 60–88 % yield. Subsequently, some di(hetero)arylamines were derivatized to the respective tertiary amines via easy N-alkylation and N-alkenylation reactions at 55–82 % yields. Finally, the Lipinski parameters and the total antioxidant activity of the new series of secondary arylamines were also evaluated.
Graphical abstract



Similar content being viewed by others
References
Ali A, Corrêa A, Alves D, Schpector JZ, Westermann B, Ferreira MAB, Paixão MW (2014) Chem Commun 50:11926
Sahu PK, Sahu PK, Lal J, Thavaselvam D, Agarwal DD (2012) Med Chem Res 21:3826
Ali R, Siddiqui N (2013) J Chem 2013:1
Reddy DRS, Kumar KH (2013) World J Pharm Pharm Sci 3:778
Ames BN, Shigenaga MK, Hagen TM (1993) Proc Natl Acad Sci 90:7915
Tyagi YK, Kumar A, Raj HG, Vohra P, Gupta G, Kumari R, Kumar P, Gupta RK (2005) Eur J Med Chem 40:413
Karegoudar P, Karthikeyan MS, Prasad DJ, Mahalinga M, Holla BS, Kumari NS (2008) Eur J Med Chem 43:261
Zanatta N, Amaral SS, Santos JM, Mello DL, Fernandes LF, Bonacorso HG, Martins MAP, Andricopulo AD, Borchardt DM (2008) Bioorg Med Chem 16:10236
Alonso C, Marigorta EM, Rubiales G, Palacios F (2015) Chem Rev 115:1847
Nenajdenko VG, Balenkova ES (2011) Arkivoc i:246
Bonacorso HG, Cavinatto S, Moraes MC, Pittaluga EP, Peroza LR, Venturini T, Alves SH, Stefanello ST, Soares FAA, Martins MAP, Zanatta N, Frizzo CP (2015) J Braz Chem Soc 26:2346
Bonacorso HG, Rodrigues MB, Rosa WC, Silva LB, Frizzo CP, Zanatta N, Martins MAP (2015) J Fluor Chem 178:296
Bonacorso HG, Paim GR, Porte LMF, Pittaluga EP, Cavinatto S, Meyer AR, Martins MAP, Zanatta N (2014) J Heterocycl Chem 41:1171
Bonacorso HG, Moraes MC, Wiethan CW, Luz FM, Meyer AR, Zanatta N, Martins MAP (2013) J Fluor Chem 156:112
Bonacorso HG, Navarini J, Wiethan CW, Bortolotto GP, Paim GR, Cavinatto S, Martins MAP, Zanatta N (2011) J Fluor Chem 132:160
Shikhaliev KhS, Falaleev AV, Kryl’skii DV (2003) Izbrannye metody sinteza i modifikatsii geterotsiklov (Selected Methods of Synthesis and Modification of Heterocycles), vol 1. Moscow, IBS, p 450
Katritzky A, Yousaf TI (1986) Can J Chem 64:2087
Chen H, Bai J, Jiao L, Guo Z, Yin Q, Li X (2009) Bioorg Med Chem 17:3980
Zhang HQ, Xia Z, Vasudevan A, Djuric SW (2006) Tetrahedron Lett 47:4881
Tavares FX, Boucheron JA, Dickerson SH, Griffin RJ, Preugschat F, Thomson SA, Wang TY, Zhou H-Q (2004) J Med Chem 47:4716
Byth KF, Culshaw JD, Green S, Oakes SE, Thomas AP (2004) Bioorg Med Chem Lett 14:2245
Bonacorso HG, Bortolotto GP, Navarini J, Porte LMF, Wiethan CW, Zanatta N, Martins MAP, Flores AFC (2010) J Fluor Chem 131:1297
Bonacorso HG, Calheiro TP, Zanatta N, Martins MAP (2015) Monatsh Chem 146:1851
Bala M, Verma PK, Sharma D, Kumar N, Singh B (2015) Mol Divers 19:263
Mohit D, Verma SM, Meena RP (2012) PDA J Pharm Sci Technol 4:873
Hojo M, Masuda R, Kokuryo Y, Shioda H, Matsuo S (1976) Chem Lett 5:499
Masaru H, Ryōichi M, Syūhei S, Makoto T (1986) Synthesis 1016
Colla A, Martins MAP, Clar G, Krimmer S, Fischer P (1991) Synthesis 483
Martins MAP, Guarda EA, Frizzo CP, Moreira DN, Marzari MRB, Zanatta N, Bonacorso HG (2009) Catal Lett 130:93
Flores AFC, Siqueira GM, Freitag R, Zanatta N, Martins MAP (1994) Quim Nova 17:298
Kamitori Y, Hojo M, Masuda R, Fujitani T, Kobuchi T, Nishigaki T (1986) Synthesis 340
Hojo M, Masuda R, Kamitori Y (1976) Tetrahedron Lett 1009
Siqueira GM, Flores AFC, Clar G, Zanatta N, Martins MAP (1994) Quim Nova 17:24
Queiroz MJRP, Calhelha RC, Vale-Silva LA, Pinto E, Nascimento MSJ (2010) Eur J Med Chem 45:5732
Queiroz MJRP, Peixoto D, Calhelha RC, Soares P, Santos T, Lima RT, Campos JF, Abreu RMV, Ferreira ICFR, Vasconcelos MH (2013) Eur J Med Chem 69:855
Lipinski CA, Lombardo F, Dominy BW, Freeney PJ (1997) Adv Drug Deliv Rev 23:3
Yamazaki T, Taguchi T, Ojima I (2009) Fluorine in medicinal chemistry and chemical biology. Wiley, Chichester, p 3
Luzina EL, Popov AV (2013) J Fluor Chem 148:41
Prieto P, Pineda M, Aguilar M (1999) Anal Biochem 269:337
Brand-Williams W, Cuvelier ME, Berset C (1995) LWT Food Sci Technol 28:25
Zhou ZZ, Yanh GF (2006) Bioorg Med Chem 14:8666
Acknowledgments
The authors thank the Coordination for Improvement of Higher Education Personnel (CAPES) for the fellowships, as well as the National Council for Scientific and Technological Development (CNPq) for financial support (Process numbers 306.883/2015-5 and 471.269/2013-1-CNPq/Universal).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonacorso, H.G., Calheiro, T.P., Rodrigues, M.B. et al. Eco-friendly synthesis and antioxidant activity of new trifluoromethyl-substituted N-(pyrimidin-2-yl)benzo[d]thiazol-2-amines and some N-derivatives. Monatsh Chem 147, 2185–2194 (2016). https://doi.org/10.1007/s00706-016-1829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1829-0