Abstract
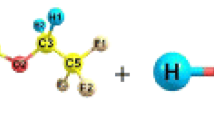
Detailed theoretical investigation has been carried out on the mechanism, kinetics, and thermochemistry of the gas-phase reactions of CH3CH2CH2C(O)OCH2CH3 with OH radicals using a modern DFT functional. Reaction profiles are modeled with the formation of pre- and post-reactive complexes. Energetic calculations are made at M06-2X/6–31+G(d,p) level of theory. Intrinsic reaction coordinate calculation has also been performed to confirm the smooth transition from the reactant to product through the respective transition state. It has been established that the reaction proceeded via H-atom abstraction from the –CH2 position of ethyl butyrate. The calculated rate constant using canonical transition state theory is found to be in a reasonable agreement with the experimental data. Using group-balanced isodesmic reactions, the standard heats of formation of ethyl butyrate and radicals generated by H-atom abstraction are also reported for the first time. The branching ratios of the different reaction channels are also determined. The estimated atmospheric life time of ethyl butyrate is found to be 2.37 days.
Graphical abstract

.






Similar content being viewed by others
References
Liang P, Mu Y, Daele V, Mellouki A (2010) Chem Phys Chem 11:4097
Klosowski G, Czuprynski B (2006) J Food Eng 72:242
Chen LG, Mai BX, Bi XH, Chen SJ, Wang XM, Ran Y, Luo XJ, Sheng GY, Fu JM, Zeng E (2006) Environ Sci Technol 40:1190
Smith DF, McIver CD, Kleindienst TE (1995) Int J Chem Kinet 27:453
Bravo I, Dıaz-de-Mera Y, Aranda A, Moreno E, Nutt DR, Marston G (2011) Phys Chem Chem Phys 13:17185
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2010) Environ Sci Technol 44:2354
Oyaro N, Sellevag SR, Neilsen CJ (2004) Environ Sci Technol 38:5567
Jordan A, Frank H (1999) Environ Sci Technol 33:522
Ninomiya Y, Kawasaki M, Guschin A, Molina LT, Molina MJ, Wallington TJ (2000) Environ Sci Technol 34:2973
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2008) Chem Phys Lett 453:18
Gour NK, Deka RC, Singh HJ, Mishra BK (2014) J Fluorine Chem 160:64
Deka RC, Mishra BK (2014) Chem Phys Lett 595:43
Blanco MB, Rivela C, Teruel MA (2013) Chem Phys Lett 578:33
Chakrabartty AK, Mishra BK, Bhattacharjee D, Deka RC (2013) Mol Phys 111:860
Mishra BK, Chakrabartty AK, Deka RC (2013) J Mol Model 19:2189
Mishra BK, Chakrabartty AK, Deka RC (2014) Struct Chem 25:463
Pires-Cabral P, da Fonseca MMR, Ferreira-Dias S (2010) Biochem Eng J 48:246
Pires-Cabral P, da Fonseca MMR, Ferreira-Dias S (2009) Biochem Eng J 43:327
Annapurna Devi N, Subbarao CV, Dharwal SJ, Narasimha Rao M (2011) Int J Tech 1:72
Wallington TJ, Dagaud P, Liu R, Kurylo M (1988) Int J Chem Kinet 20:177
Ferrari C, Roche A, Jacob V, Foster P, Baussand P (1996) Int J Chem Kin 28:609
Hammond GS (1955) J Am Chem Soc 77:334
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Chase MW Jr (1998) NIST-JANAF thermochemical tables, 4th edn. J Phys Chem Ref Data 9:1
El-Nahas AM, Navarro MV, Simmie JM, Bozzelli JW, Curran HJ, Dooley S, Metcalfe W (2007) J Phys Chem A 111:3727
Lide (ed) (2008) CRC handbook of chemistry and physics, 89th edn. CRC Press, New York
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, New Delhi
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221
Brown RL (1981) J Res Natl Bur Stand 86:357
Xiao R, Noerpel M, Luk HL, Wei Z, Spinney R (2014) Int J Quant Chem 114:74
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) J Phys Chem A 108:2666
Atkinson R (1997) J Phys Chem Ref Data 26:215
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215
Mishra BK, Lily M, Chakrabartty AK, Bhattacharjee D, Deka RC, Chandra AK (2014) New J Chem. doi:10.1039/C3NJ01408H
Lily M, Mishra BK, Chandra AK (2014) J Fluorine Chem 161:51
Mandal D, Sen K, Das AK (2012) J Phys Chem A 116:8382
Dinadayalane TC, Paytakov G, Leszczynski J (2013) J Mol Model 19:2855
Balaganesh M, Rajakumar B (2014) J Mol Graph Model 48:60
Dash MR, Rajakumar B (2014) Chem Phys Lett 597:75
Sandhiya L, Kolandaivel P, Senthilkumar K (2012) Struct Chem 23:1475
Mandal D, Sahu C, Bagchi S, Das AK (2013) J Phys Chem A 117:3739
Chakrabartty AK, Mishra BK, Bhattacharjee D, Deka RC (2013) J Fluorine Chem 154:60
Mishra BK, Lily M, Chakrabartty AK, Deka RC, Chandra AK (2014) J Fluorine Chem 159:57
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, DanielsAD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B.01. Gaussian Inc, Wallingford
Acknowledgments
BKM is thankful to University Grant Commission (UGC), New Delhi for providing Dr. D. S. Kothari Post-doctoral Fellowship. HJS and NKG are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for providing financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gour, N.K., Deka, R.C., Singh, H.J. et al. Theoretical study on the gas-phase reactions of ethyl butyrate with OH radicals at 298 K. Monatsh Chem 145, 1759–1767 (2014). https://doi.org/10.1007/s00706-014-1255-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1255-0