Abstract
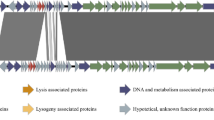
Bacteriophages are recognized as major mortality agents of microbes, among them intracellular marine rickettsiales-like bacteria. Recently, a phage hyperparasite of Candidatus Xenohaliotis californiensis (CXc) has been described. This bacterium is considered the causal agent of Withering Syndrome (WS) which is a chronic and potentially lethal disease of abalone species from California, USA and the peninsula of Baja California, Mexico. This hyperparasite which infects CXc could be used as a biocontrol agent for WS. Therefore, it is necessary to obtain genomic information to characterize this phage. In this study, the first complete genome sequence of a novel phage, Xenohaliotis phage (pCXc) was determined. The complete genome of pCXc from red abalone (Haliotis rufescens) is 35,728 bp, while the complete genome of pCXc from yellow abalone (Haliotis corrugata) is 35,736 bp. Both phage genomes consist of double-stranded DNA with a G + C content of 38.9%. In both genomes 33 open reading frames (ORFs) were predicted. Only 10 ORFs encode proteins that have identifiable functional homologues. These 10 ORFs were classified by function, including structural, DNA replication, DNA packaging, nucleotide transport and metabolism, life cycle regulation, recombination and repair, and additional functions. A PCR method for the specific detection of pCXc was developed. This information will help to understand a new group of phages that infect intracellular marine rickettsiales-like bacteria in mollusks.


Similar content being viewed by others
Change history
05 February 2018
Unfortunately, the family name of the co-author Roberto Cruz-Flores was incorrectly published in the original publication and corrected here by this correction. The original article has been corrected.
References
Mizuno CM, Rodriguez-Valera F, Kimes NE, Ghai R (2013) Expanding the marine virosphere using metagenomics. PLoS Genet 9:e1003987. https://doi.org/10.1371/journal.pgen.1003987
Getchell RG, Smolowitz RM, McGladdery SE, Bower SM (2016) Diseases and parasites of scallops. In: Shumway SE, Parsons JG (eds) Dev. Aquac. Fish. Sci., 3rd edn. Elsevier Ltd, Oxford, pp 425–467
Wang W (2011) Bacterial diseases of crabs: a review. J Invertebr Pathol 106:18–26. https://doi.org/10.1016/j.jip.2010.09.018
Fryer JL, Lannan CN (1996) Rickettsial infections of fish. Annu Rev Fish Dis 6:3–13. https://doi.org/10.1016/S0959-8030(96)90002-2
Cáceres-Martínez J, Tinoco-Orta GD (2001) Symbionts of cultured red abalone Haliotis Rufescens from Baja California, Mexico. J Shellfish Res 20:875–881
Friedman CS, Andree KB, Beauchamp KA et al (2000) “Candidatus Xenohaliotis californiensis”, a newly described pathogen of abalone, Haliotis spp., along the west coast of North America. Int J Syst Evol Microbiol 50:847–855. https://doi.org/10.1099/00207713-50-2-847
Buchanan JS (1978) Cytological studies on a new species of rickettsia found in association with a phage in the digestive gland of the marine bivalve mollusc, Tellina tenuis (da Costa). J Fish Dis 1:27–43. https://doi.org/10.1111/j.1365-2761.1978.tb00003.x
Wen C-M, Kou G-H, Chen S-N (1994) Rickettsiaceae-like Microorganisms in the Gill and Digestive Gland of the Hard Clam, Meretrix lusoria Röding. J Invertebr Pathol 64:138–142. https://doi.org/10.1006/jipa.1994.1082
Comps M, Tigé G (1999) Procaryotic infections in the mussel Mytilus galloprovinciallis and in its parasite the turbellarian Urastoma cyprinae. Dis Aquat Organ 38:211–217. https://doi.org/10.3354/dao038211
Sun J, Wu X (2004) Histology, ultrastructure, and morphogenesis of a rickettsia-like organism causing disease in the oyster, Crassostrea ariakensis Gould. J Invertebr Pathol 86:77–86. https://doi.org/10.1016/j.jip.2004.04.004
Friedman CS, Crosson LM (2012) Putative phage hyperparasite in the rickettsial pathogen of Abalone, “Candidatus Xenohaliotis californiensis”. Microb Ecol 64:1064–1072. https://doi.org/10.1007/s00248-012-0080-4
Cruz-Flores R, Cáceres-Martínez J, Muñoz-Flores M et al (2016) Hyperparasitism by the bacteriophage (Caudovirales) infecting Candidatus Xenohaliotis californiensis (Rickettsiales-like prokaryote) parasite of wild abalone Haliotis fulgens and Haliotis corrugata from the Peninsula of Baja California, Mexico. J Invertebr Pathol 140:58–67. https://doi.org/10.1016/j.jip.2016.09.001
Cruz-Flores R, Cáceres-Martínez J (2016) The hyperparasite of the rickettsiales-like prokaryote, Candidatus Xenohaliotis californiensis has morphological characteristics of a Siphoviridae (Caudovirales). J Invertebr Pathol 133:8–11. https://doi.org/10.1016/j.jip.2015.11.006
Friedman CS, Wight N, Crosson LM et al (2014) Reduced disease in black abalone following mass mortality: phage therapy and natural selection. Front Microbiol 5:1–10. https://doi.org/10.3389/fmicb.2014.00078
Langevin SA, Burge CA, Crosson ML et al (2015) Sequence analysis of the phage infecting the Withering Syndrome Rickettsia reveals a novel bacteriophage. In: Abstr. Tech. Pap. Present. 107th Annu. Meet. Natl. Shellfish. Assoc. Monterey, California, March 22–26, 2015, pp 650–651
Cruz-Flores R, Cáceres-Martínez J, Vásquez-Yeomans R (2015) A novel method for separation of Rickettsiales-like organism “Candidatus Xenohaliotis californiensis” from host abalone tissue. J Microbiol Methods 115:79–82. https://doi.org/10.1016/j.mimet.2015.05.021
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Delcher AL, Bratke KA, Powers EC, Salzberg SL (2007) Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23:673–679. https://doi.org/10.1093/bioinformatics/btm009
Besemer J, Borodovsky M (2005) GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res. https://doi.org/10.1093/nar/gki487
Lowe TM, Eddy SR (1996) TRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964. https://doi.org/10.1093/nar/25.5.0955
Schattner P, Brooks AN, Lowe TM (2005) The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33:686–689. https://doi.org/10.1093/nar/gki366
Zhuang J, Cai G, Lin Q et al (2010) A bacteriophage-related chimeric marine virus infecting abalone. PLoS One 5:e13850. https://doi.org/10.1371/journal.pone.0013850
Parratt SR, Laine A-L (2016) The role of hyperparasitism in microbial pathogen ecology and evolution. ISME J 10:1815–1822. https://doi.org/10.1038/ismej.2015.247
Taylor DR, Jarosz AM, Fulbright DW, Lenski RE (1998) The acquisition of hypovirulence in host-pathogen systems with three trophic levels. Am Nat 151:343–355. https://doi.org/10.1086/286123
Acknowledgements
This study was co-financed by Centro de Investigación Científica y de Educación Superior de Ensenada and Instituto de Sanidad Acuícola, A.C. We would like to thank Dr. Rebeca Vásquez-Yeomans for her valuable revisions, comments and support on the manuscript. A special thanks to Yanet Guerrero and Olga Callejas-Negrete for technical assistance with sample processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was co-financed by Centro de Investigación Científica y Educación Superior de Ensenada (project number 623106) and Instituto de Sanidad Acuícola, A. C. (project number Ab8758-2).
Conflict of interest
Roberto Cruz-Flores declares that there is not conflict of interest. Jorge Cáceres-Martínez declares that there is not conflict of interest. Miguel Ángel Del Río-Portilla declares that there is not conflict of interest. Alexei F. Licea-Navarro declares that there is not conflict of interest. Ricardo Alberto González-Sánchez declares that there is not conflict of interest. Abraham Guerrero declares that there is not conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Handling Editor: T. K. Frey.
The original version of this article was revised: The family name of the co-author Roberto Cruz-Flores was incorrectly published in the original publication and corrected here.
Rights and permissions
About this article
Cite this article
Cruz-Flores, R., Cáceres-Martínez, J., Del Río-Portilla, M.Á. et al. Complete genome sequence of a phage hyperparasite of Candidatus Xenohaliotis californiensis (Rickettsiales) – a pathogen of Haliotis spp (Gasteropoda). Arch Virol 163, 1101–1104 (2018). https://doi.org/10.1007/s00705-018-3703-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3703-3