Abstract
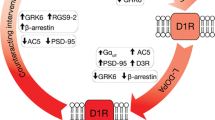
The role of cyclic ADP-ribose (cADPR) as a second messenger and modulator of the mTOR pathway downstream of dopamine (DA) receptors and/or CD38 was re-examined in the mouse. ADP-ribosyl activity was low in the membranes of neonates, but DA stimulated it via both D1- and D2-like receptors. ADP-ribosyl cyclase activity increased significantly during development in association with increased expression of CD38. The cADPR binding proteins, FKBP12 and FKBP12.6, were expressed in the adult mouse striatum. The ratio of phosphorylated to non-phosphorylated S6 kinase (S6K) in whole mouse striatum homogenates decreased after incubation of adult mouse striatum with extracellular cADPR for 5 min. This effect of cADPR was much weaker in MPTP-treated Parkinson’s disease model mice. The inhibitory effects of cADPR and rapamycin were identical. These data suggest that cADPR is an endogenous inhibitor of the mTOR signaling pathway downstream of DA receptors in the mouse striatum and that cADPR plays a certain role in the brain in psychiatric and neurodegenerative diseases.






Similar content being viewed by others
References
Barker RA, Drouin-Ouellet J, Parmar M (2015) Cell-based therapies for Parkinson disease—past insights and future potential. Nat Rev Neurol 11(9):492–503
Bergson C, Levenson R, Goldman-Rakic PS, Lidow MS (2003) Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. Trends Pharmacol Sci 24(9):486–492
Bockaert J, Marin P (2015) mTOR in brain physiology and pathologies. Physiol Rev 95(4):1157–1187
Buszczak M, Signer RA, Morrison SJ (2014) Cellular differences in protein synthesis regulate tissue homeostasis. Cell 159(2):242–251
Ceni C, Muller-Steffner H, Lund F, Pochon N, Schweitzer A, De Waard M, Schuber F, Villaz M, Moutin MJ (2003) Evidence for an intracellular ADP-ribosyl cyclase/NAD+-glycohydrolase in brain from CD38-deficient mice. J Biol Chem 278(42):40670–40678
De Flora A, Zocchi E, Guida L, Franco L, Bruzzone S (2004) Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann N Y Acad Sci 1028:176–191
Frégeau MO, Carrier M, Guillemette G (2013) Mechanism of dopamine D2 receptor-induced Ca2+ release in PC-12 cells. Cell Signal 25(12):2871–2877
Fukuda K, Higashida H, Kubo T, Maeda A, Akiba I, Bujo H, Mishina M, Numa S (1988) Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature 335(6188):355–358
Furuya S, Sawada M, Nagatsu T, Suzuki O, Higashida H (1985) Localization of [3H]serotonin in neuroblastoma x glioma hybrid cells. Brain Res 361(1–2):77–90
Graeff R, Lee HC (2002) A novel cycling assay for nicotinic acid-adenine dinucleotide phosphate with nanomolar sensitivity. Biochem J 367(Pt 1):163–168
Greengard P (2001) The neurobiology of slow synaptic transmission. Science 294(5544):1024–1030
Hausch F (2015) FKBPs and their role in neuronal signaling. Biochim Biophys Acta 1850(10):2035–2040
Higashida H, Brown DA (1986) Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. Nature 323(6086):333–335
Higashida H, Yokoyama S, Hashii M, Taketo M, Higashida M, Takayasu T, Ohshima T, Takasawa S, Okamoto H, Noda M (1997) Muscarinic receptor-mediated dual regulation of ADP-ribosyl cyclase in NG108-15 neuronal cell membranes. J Biol Chem 272(50):31272–31277
Higashida H, Egorova A, Higashida C, Zhong ZG, Yokoyama S, Noda M, Zhang JS (1999) Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J Biol Chem 274(47):33348–33354
Higashida H, Zhang J, Hashii M, Shintaku M, Higashida C, Takeda Y (2000) Angiotensin II stimulates cyclic ADP-ribose formation in neonatal rat cardiac myocytes. Biochem J 352(Pt 1):197–202 (Erratum in: Biochem J 2001 354(Pt 3):727)
Higashida H, Hashii M, Yokoyama S, Hoshi N, Asai K, Kato T (2001a) Cyclic ADP-ribose as a potential second messenger for neuronal Ca2+ signaling. J Neurochem 76(2):321–331
Higashida H, Hashii M, Yokoyama S, Hoshi N, Chen XL, Egorova A, Noda M, Zhang JS (2001b) Cyclic ADP-ribose as a second messenger revisited from a new aspect of signal transduction from receptors to ADP-ribosyl cyclase. Pharmacol Ther 90(2–3):283–296
Higashida H, Hossain KZ, Takahagi H, Noda M (2002) Measurement of adenylyl cyclase by separating cyclic AMP on silica gel thin-layer chromatography. Anal Biochem 308(1):106–111
Higashida H, Zhang JS, Mochida S, Chen XL, Shin Y, Noda M, Hossain KZ, Hoshi N, Hashii M, Shigemoto R, Nakanishi S, Fukuda Y, Yokoyama S (2003) Subtype-specific coupling with ADP-ribosyl cyclase of metabotropic glutamate receptors in retina, cervical superior ganglion and NG108-15 cells. J Neurochem 85(5):1148–1158
Higashida H, Salmina AB, Olovyannikova RY, Hashii M, Yokoyama S, Koizumi K, Jin D, Liu HX, Lopatina O, Amina S, Islam MS, Huang JJ, Noda M (2007) Cyclic ADP-ribose as a universal calcium signal molecule in the nervous system. Neurochem Int 51(2–4):192–199
Higashida C, Islam MS, Kamimura S, Inoue T, Jin D, Zhang J, Hashii M, Liang M, Zhong J, Hori O, Fukunaga K, Okamoto H, Graeff R, Lee HC, Higashida H (2013) Dopamine-induced regulation and deregulation of the catabolism of cyclic ADP-ribose, an intrinsic mTOR signal inhibitor, during development in the rodent striatum. Messenger 2(1):33–43
Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33(2):67–75
Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E (2008) Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron 60(5):832–845
Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H (2003) AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci 6(6):564–571
Hua SY, Tokimasa T, Takasawa S, Furuya Y, Nohmi M, Okamoto H, Kuba K (1994) Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron 12(5):1073–1079
Iversen SD, Iversen LL (2007) Dopamine: 50 years in perspective. Trends Neurosci 30(5):188–193
Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H (2007) CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446(7131):41–45
Kim UH (2014) Multiple Enzymatic activities of CD38 for Ca2+ signaling. Messenger 3(1):6–14
Lee HC (2012) The Cyclic ADP-ribose/NAADP/CD38-signaling pathway: past and present. Messenger 1(1):16–33
Lipton JO, Sahin M (2014) The neurology of mTOR. Neuron 84(2):275–291
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78(1):189–225
Nagatsu T (2007) The catecholamine system in health and disease -Relation to tyrosine 3-monooxygenase and other catecholamine-synthesizing enzymes. Proc Jpn Acad Ser B Phys Biol Sci 82(10):388–415
Nagatsu T, Nagatsu I (2016) Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): historical overview and future prospects. J Neural Transm (Vienna) 123:1255–1278
Nagatsu T, Nakano T, Kato T, Higashida H (1981) Expression of A and B types of monoamine oxidase in neuroblastoma hybrid cells. Neurochem Int 3(2):137–142
Nakano T, Nagatsu T, Higashida H (1985) Expression of A and B types of monoamine oxidase in differentiated neuroblastoma hybrid cells. J Neurochem 44(3):755–758
Nakano T, Saito S, Higashida H, Kojima K, Nagatsu T (1986) Assignment of A and B types of monoamine oxidase in NCB20 hybrid cells to those of the parental cells by peptide mapping. J Neurochem 46(3):686–694
Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H (1997) Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem 272(6):3133–3136
Okamoto H, Takasawa S, Sugawara A (2014) The CD38-cyclic ADP-ribose system in mammals: historical background, pathophysiology and perspective. Messenger 3(1):27–34
Roohi A, Hojjat-Farsangi M (2016) Recent advances in targeting mTOR signaling pathway using small molecule inhibitors. J Drug Target 3:1–13
Salmina AB, Lopatina O, Ekimova MV, Mikhutkina SV, Higashida H (2010) CD38/cyclic ADP-ribose system: a new player for oxytocin secretion and regulation of social behaviour. J Neuroendocrinol 22(5):380–392
Santini E, Heiman M, Greengard P, Valjent E, Fisone G (2009) Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal 2(80):ra36
Seeman P, Van Tol HH (1994) Dopamine receptor pharmacology. Trends Pharmacol Sci 15(7):264–270
Sukhbaatar N, Hengstschläger M, Weichhart T (2016) mTOR-mediated regulation of dendritic cell differentiation and function. Trends Immunol. doi:10.1016/j.it.2016.08.009
Sutton LP, Caron MG (2015) Essential role of D1R in the regulation of mTOR complex1 signaling induced by cocaine. Neuropharmacology 99:610–619. doi:10.1016/j.neuropharm.2015.08.024
Suzuki O, Hattori H, Sawada M, Nagatsu T, Miki N, Higashida H (1983) Serotonin in neuroblastoma x glioma NG108-15 hybrid cells. Neurochem Int 5(5):599–601
Thomson AW, Turnquist HR, Raimondi G (2009) Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9(5):324–337
Tramutola A, Lanzillotta C, Di Domenico F (2016) Targeting mTOR to reduce Alzheimer-related cognitive decline: from current hits to future therapies. Expert Rev Neurother 17:33–45
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162(4):712–725
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Yano K, Higashida H, Inoue R, Nozawa Y (1984) Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem 259(16):10201–10207
Zhang JS, Jin D, Higashida H (2005) Acetylcholine stimulates cyclic ADP-ribose formation via M1 muscarinic receptors in rat superior cervical ganglion. Biochem Biophys Res Commun 335(3):920–924
Zhang X, Tallini YN, Chen Z, Gan L, Wei B, Doran R, Miao L, Xin HB, Kotlikoff MI, Ji G (2009) Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not beta-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res 84(2):253–262
Acknowledgements
This work was supported by Grant-in-aid from by the Industry-Academia Collaborative R&D Programs (COI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Higashida, H., Kamimura, Sy., Inoue, T. et al. Cyclic ADP-ribose as an endogenous inhibitor of the mTOR pathway downstream of dopamine receptors in the mouse striatum. J Neural Transm 125, 17–24 (2018). https://doi.org/10.1007/s00702-016-1666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1666-7