Abstract
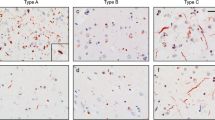
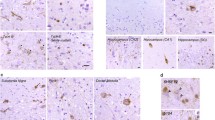
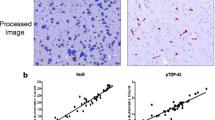
The BrainNet Europe consortium assessed the reproducibility in the assignment of the type of frontotemporal lobar degeneration (FTLD) with TAR DNA-binding protein (TDP) 43 following current recommendations. The agreement rates were influenced by the immunohistochemical (IHC) method and by the classification strategy followed. p62-IHC staining yielded good uniform quality of stains, but the most reliable results were obtained implementing specific Abs directed against the hallmark protein TDP43. Both assessment of the type and the extent of lesions were influenced by the Abs and by the quality of stain. Assessment of the extent of the lesions yielded poor results repeatedly; thus, the extent of pathology should not be used in diagnostic consensus criteria. Whilst 31 neuropathologists typed 30 FTLD-TDP cases, inter-rater agreement ranged from 19 to 100 per cent, being highest when applying phosphorylated TDP43/IHC. The agreement was highest when designating Type C or Type A/B. In contrast, there was a poor agreement when attempting to separate Type A or Type B FTLD-TDP. In conclusion, we can expect that neuropathologist, independent of his/her familiarity with FTLD-TDP pathology, can identify a TDP43-positive FTLD case. The goal should be to state a Type (A, B, C, D) or a mixture of Types (A/B, A/C or B/C). Neuropathologists, other clinicians and researchers should be aware of the pitfalls whilst doing so. Agreement can be reached in an inter-laboratory setting regarding Type C cases with thick and long neurites, whereas the differentiation between Types A and B may be more troublesome.



Similar content being viewed by others
References
Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopoulou P, Kovacs GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H (2006) Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe consortium. J Neuropathol Exp Neurol 65:740–757
Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Stadelmann C, Streichenberger N, Tagliavini F, Kretzschmar H (2008a) Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol 115(5):533–546
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovács GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H (2008b) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe consortium. Brain Pathol 18(4):484–496
Alafuzoff I, Parkkinen L, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kamphorst W, King A, Korkolopoulou P, Kovács GG, Larionov S, Meyronet D, Monoranu C, Morris J, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H, BrainNet Europe Consortium (2008c) Assessment of alpha-synuclein pathology: a study of the BrainNet Europe consortium. J Neuropathol Exp Neurol 67(2):125–143
Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Parchi P, Parkkinen L, Patsouris E, Roggendorf W, Rozemuller A, Stadelmann-Nessler C, Streichenberger N, Thal DR, Kretzschmar H (2009a) Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe consortium. Acta Neuropathol 117(6):635–652
Alafuzoff I, Thal DR, Arzberger T, Bogdanovic N, Al-Sarraj S, Bodi I, Boluda S, Bugiani O, Duyckaerts C, Gelpi E, Gentleman S, Giaccone G, Graeber M, Hortobagyi T, Höftberger R, Ince P, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Nilsson T, Parchi P, Patsouris E, Pikkarainen M, Revesz T, Rozemuller A, Seilhean D, Schulz-Schaeffer W, Streichenberger N, Wharton SB, Kretzschmar H (2009b) Assessment of beta-amyloid deposits in human brain: a study of the BrainNet Europe consortium. Acta Neuropathol 117(3):309–320
Arai T, Nonaka T, Hasegawa M, Akiyama H, Yoshida M, Hashizume Y, Tsuchiya K, Oda T, Ikeda K (2003) Neuronal and glial inclusions in frontotemporal dementia with or without motor neuron disease are immunopositive for p62. Neurosci Lett 15:41–44
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2013) Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74:20–38
Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, Fang L, Van Deerlin VM, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2014) Sequential distribution ofpTDP-43 pathology in behavioural variant frontotempiral dementia (bvFTD). Acta Neuropathol 127:423–439
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL III, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM, Consortium for Frontotemporal Lobar Degeneration (2007a) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol 114(1):5–22
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR (2007b) TDP43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171(1):227–240
Croisier E, MRes DE, Deprez M, Goldring K, Dexter DT, Pearce RK, Graeber MB, Roncaroli F (2006) Comparative study of commercially available anti-alpha-synuclein antibodies. Neuropathol Appl Neurobiol 32(3):351–356
D’Amico F, Skarmoutsou E, Stivala F (2009) State of the art in antigen retrieval for immunohistochemistry. J Immunol Methods 341:1–18
Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP43. Acta Neuropathol 113:521–533
Gelpi E, Preusser M, Bauer G, Budka H (2007) Autopsy at 2 months after death: brain is satisfactorily preserved for neuropathology. Forensic Sci Int 168(2–3):177–182
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H (2008) Phosphorylated TDP43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64(1):60–70
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging–Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8(1):1–13
Jackson M, Lennox G, Lowe J (1996) Motor neurone disease-inclusion dementia. Neurodegeneration 5:339–350
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122(2):137–153
Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Jack CR Jr, Parisi JE, Petersen RC, Dickson DW (2014) TDP43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. PMID: 24659241 (Epub ahead of print)
Karlsson C, Karlsson MG (2011) Effects of long-term storage on the detection of proteins, DNA, and mRNA in tissue microarray slides. J Histochem Cytochem 59:1113–1121
Kauppinen T, Martikainen P, Alafuzoff I (2006) Human postmortem brain tissue and 2-mm tissue microarrays. Appl Immunohistochem Mol Morphol 14:353–359
Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N, Leisser I, Verchère J, Baron T, Alafuzoff I, Budka H, Perret-Liaudet A, Lachmann I (2012) An antibody with high reactivity for disease-associated α-synuclein reveals extensive brain pathology. Acta Neuropathol 124(1):37–50
Kuusisto E, Kauppinen T, Alafuzoff I (2008) Use of p62/SQSTM1 antibodies for neuropathological diagnosis. Neuropathol Appl Neurobiol 34(2):169–180
Lowe J, Lennox G, Jefferson D, Morrell K, McQuire D, Gray T, Landon M, Doherty FJ, Mayer RJ (1988) A filamentous inclusion body within anterior horn neurones in motor neurone disease defined by immunocytochemical localisation of ubiquitin. Neurosci Lett 94(1–2):203–210
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM (2006a) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112(5):539–549
Mackenzie IR, Shi J, Shaw CL, Duplessis D, Neary D, Snowden JS, Mann DM (2006b) Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol 112(5):551–559
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122(1):111–113
Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, Rossor MN, Hardy J, Collinge J, Revesz T, Mead S, Warren JD (2012) Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 135(Pt 3):736–750
McNicol AM, Richmond JA (1998) Optimizing immunohistochemistry: antigen retrieval and signal amplification. Histopathology 32:97–103
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on Aging; Alzheimer’s Association (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123(1):1–11
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM (2009) Phosphorylation of S409/410 of TDP43 is a consistent feature in all sporadic and familial forms of TDP43 proteinopathies. Acta Neuropathol 117(2):137–149
Pikkarainen M, Hartikainen P, Alafuzoff I (2008) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions visualized with ubiquitin-binding protein p62 immunohistochemistry. J Neuropathol Exp Neurol 67(4):280–298
Pikkarainen M, Martikainen P, Alafuzoff I (2010a) The effect of prolonged fixation time on immunohistochemical staining of common neurodegenerative disease markers. J Neuropathol Exp Neurol 69(1):40–52
Pikkarainen M, Hartikainen P, Alafuzoff I (2010b) Ubiquitinated p62-positive, TDP43-negative inclusions in cerebellum in frontotemporal lobar degeneration with TAR DNA binding protein 43. Neuropathology 2:197–199
Pikkarainen M, Hartikainen P, Soininen H, Alafuzoff I (2011) Distribution and pattern of pathology in subjects with familial or sporadic late-onset cerebellar ataxia as assessed by p62/sequestosome immunohistochemistry. Cerebellum 10(4):720–731
Pirici D, Vandenberghe R, Rademakers R, Dermaut B, Cruts M, Vennekens K, Cuijt I, Lübke U, Ceuterick C, Martin JJ, Van Broeckhoven C, Kumar-Singh S (2006) Characterization of ubiquitinated intraneuronal inclusions in a novel Belgian frontotemporal lobar degeneration family. J Neuropathol Exp Neurol 65(3):289–301
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Shi SR, Cote RJ, Taylor CR (2001) Antigen retrieval immunohistochemistry and molecular morphology in the year 2001. Appl immunohisto Mol Morphol 9:107–116
Tan RH, Shepherd CE, Kril JJ, McCann H, McGeachie A, McGinley C, Affleck A, Halliday GM (2013) Classification of FTLD-TDP cases into pathological subtypes using antibodies against phosphorylated and non-phosphorylated TDP43. Acta Neuropathol Commun 1(1):33
Acknowledgments
We thank Tarja Kauppinen and all other laboratory technicians of the BNE members for their skilful technical assistance and Meena Strömqvist for her critical reading of the manuscript. We acknowledge the following BNE centres (in alphabetical order of the cities) for contributing the material with generic data used in this study: Netherlands Brain Bank (Amsterdam), Hospital de Bellvitge/Universitat de Barcelona (Barcelona), National Institute of Psychiatry and Neurology (Budapest, OPNI) closed in 2007, Georg-August-University (Göttingen), Karolinska Institutet (Huddinge), Kuopio University Hospital (Kuopio), MRC London Neurodegenerative Disease Brain Bank Institute of Psychiatry (London), Hospices Civils de Lyon (Lyon), Istituto Nazionale Neurologico Carlo Besta (Milan), Ludwig-Maximilians-University of Munich (Munich), Medical University Vienna (Vienna) and University of Wuerzburg (Wuerzburg). This study was supported by the European Union Grant FP6: BNEII No LSHM-CT-2004-503039. TA, HK and DRT were also supported by the German ministry for education and research (BMBF; FTLDc). IA was also supported by the local Grants (ALF) from Uppsala University Hospital, Sweden. This article reflects only the authors’ views, and the community is not liable for any use that may be made of the information contained herein. The study has been authorised by the Ethics Committee of Kuopio University Hospital. DRT received consultant honorary from Simon Kucher and partners, GE-Healthcare, and Covance Laboratories, speaker honorary from GE-Healthcare and collaboration with Novartis Pharma AG.
Author information
Authors and Affiliations
Corresponding author
Additional information
BrainNet Europe: http://www.brainnet-europe.org/.
Electronic supplementary material
Below is the link to the electronic supplementary material.
702_2014_1304_MOESM1_ESM.ppt
Supplement Fig. 1 Summary of the published subtyping recommendations in tabulated form and the instructions of assessment of immunoreactive lesions (PPT 540 kb)
Rights and permissions
About this article
Cite this article
Alafuzoff, I., Pikkarainen, M., Neumann, M. et al. Neuropathological assessments of the pathology in frontotemporal lobar degeneration with TDP43-positive inclusions: an inter-laboratory study by the BrainNet Europe consortium. J Neural Transm 122, 957–972 (2015). https://doi.org/10.1007/s00702-014-1304-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1304-1