Abstract
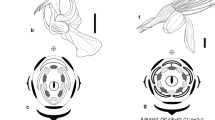
Passiflora lobata differs from most other passion flowers in that it has a tetramerous gynoecium and dorsiventral flowers. A detailed ontogenetic analysis using scanning electron microscopy revealed the following characters: tendril formation starts late, indicating an axial nature. The paired flowers show mirror symmetry, which is manifested very early in ontogeny. Five sepals initiate in a spiral followed by five petals, which are formed successively adjacent to each other. This is a rare pattern and the first report in Passifloraceae. Frequently a sixth petal primordium was found, which never develops and which could be interpreted as the first outgrowth or frill of the corona (which therefore might be interpreted as derived from the perianth). The abaxial carpel forms always in front of the first-formed sepal. The remaining three carpels are alternate with the stamens. This means that a positional change took place from the typical trimerous ovary with two carpels in front of stamens to only one antestaminal carpel in P. lobata. This shift might have opened up space for a fourth carpel. Together with the analysis of other tetramerous Passifloraceae, this study will foster the understanding of flower morphology in this family and its systematic relationships among Malpighiales.







Similar content being viewed by others
References
APG (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Bell AD (2008) Plant form. An illustrated guide to flowering plant morphology, 2nd edn. Timber Press, London
Bello MA, Hawkins JA, Rudall PJ (2007) Floral morphology and development in Quillajaceae and Surianaceae (Fabales), the species-poor relatives of Leguminosae and Polygalaceae. Ann Bot 100:1491–1505
Bernhard A (1999) Flower structure, development, and systematics in Passifloraceae and in Abatia (Flacourtiaceae). Int J Plant Sci 160:135–150
Buzato S, Franco ALM (1992) Tetrastylis ovalis: a second case of bat pollinated passionflower (Passifloraceae). Plant Syst Evol 181:261–267
Calonje M, Cubas P, Martinez-Zapater JM, Carmona MJ (2004) Floral meristem identity genes are expressed during tendril development in grapevine. Plant Physiol 135:1491–1501
Cardoso MZ (2008) Herbivore handling of a plant’s trichome: the case of Heliconius chrithonia (L.) (Lepidoptera: Nymphalidae) and Passiflora lobata (Killip) Hutch. (Passifloraceae). Neotrop Entomol 37:247–252
Caze ALR, Mäder G, Bonatto SL, Freitas LB (2013) A molecular systematic analysis of Passiflora ovalis and Passiflora contracta (Passifloraceae). Phytotaxa 132:39–46
De Candolle AP (1813) Théorie élémentaire de la botanique. Paris
De Wilde WJJO (1974) The genera of tribe Passifloreae (Passifloraceae) with special reference to flower morphology. Blumea 22:37–50
Drinnan A, Carrucan A (2005) The ontogenetic basis for floral diversity in Agonis, Leptospermum and Kunzea (Myrtaceae). Plant Syst Evol 251:71–88
Eichler AW (1878) Blüthendiagramme. 2. Teil. W. Engelmann, Leipzig
Endress PK (1994) Diversity and evolutionary biology of tropical flowers. Cambridge University Press, Cambridge
Endress PK (1999) Symmetry in flowers: diversity and evolution. Int J Plant Sci 160:S3–S23
Endress PK, Matthews ML (2006) Elaborate petals and staminodes in eudicots: diversity, function and evolution. Org Div Evol 6:257–293
Endress PK, Davis CC, Matthews ML (2013) Advances in the floral structural characterization of the major subclades of Malpighiales, one of the largest orders of flowering plants. Ann Bot 111:969–985
Feuillet C (2011) Two new species of Dilkea subgenus Dilkea (Passifloraceae) from Loreto, Peru. PhytoKeys 2:1–8
Feuillet C, MacDougal JM (2004) A new infrageneric classification of Passiflora L. (Passifloraceae). Passiflora 13:34–38
Feuillet C, MacDougal JM (2007) Passifloraceae. In: Kubizki K (ed) The families and genera of vascular plants. Springer, Berlin, pp 270–281
Gentry AH (1976) Additional Panamanian Passifloraceae. Ann Missouri Bot Gard 63:341–345
Hansen AK, Gilbert LE, Simpson BB, Downie SR, Cervi AC, Jansen RK (2006) Phylogenetic relationships and chromosome number evolution in Passiflora. Syst Bot 31:138–150
Harms H (1893) Passifloraceae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien. W. Engelmann, Leipzig, pp 69–94
Harms H (1897) Zur Morphologie der Ranken und Blütenstände bei den Passifloraceen. Bot Jahrb 24:163–178
Harms H (1931) Eine neue Gattung der Flacourtiaceae. Notizbl königl bot Gart Berlin 11:146–149
Harms H (1932) Zur Kenntnis der Gattung Ancistrothyrsus. Notizbl königl bot Gart Berlin 11:598–600
Hemingway CA, Christensen AR, Malcomber ST (2011) B- and C-class gene expression during corona development of the blue passionflower (Passiflora caerulea, Passifloraceae). Am J Bot 98:923–934
Hofmeister W (1868) Allgemeine Morphologie der Gewächse. W. Engelmann, Leipzig
Killip EP (1926) Tetrastylis, a genus of Passifloraceae. J Wash Acad Sci 16:365–369
Killip EP (1938) The American species of Passifloraceae. Bot Ser Field Mus Nat Hist 19:1–613
Kirchoff BK (2003) Shape matters: Hofmeister’s rule, primordium shape, and flower orientation. Int J Plant Sci 164:505–517
Krosnick SE, Freudenstein JV (2005) Monophyly and floral character homology of old world Passiflora (subgenus Decaloba: supersection Disemma). Syst Bot 30:139–152
Krosnick SE, Harris EM, Freudenstein JV (2006) Patterns of anomalous floral development in the Asian Passiflora (subgenus Decaloba: supersection Disemma). Am J Bot 93:620–636
Krosnick SE, Ford AJ, Freudenstein JV (2009) Taxonomic revision of Passiflora subgenus Tetrapathea including the monotypic genera Hollrungia and Tetrapathea (Passifloraceae), and a new species of Passiflora. Syst Bot 34:375–385
Krosnick SE, Porter-Utley KE, Macdougal JM, Jorgensen PM, McDade LA (2013) New insights into the evolution of Passiflora subgenus Decaloba (Passifloraceae): phylogenetic relationships and morphological synapomorphies. Syst Bot 38:692–713
Levin DA (1973) The role of trichomes in plant defense. Quart Rev Biol 48:3–15
Lindman CAM (1906) Zur Kenntnis der Corona einiger Passifloren. Botaniska Studier, tellägnade F.R. Kjellman den 4 November 1906. Almquist & Wiksells Boktryckerei-A.-B., Uppsala
MacDougal JM (1986) A new combination in Passifloraceae. Phytologia 60:446
MacDougal JM (1994) Revision of Passiflora subgenus Decaloba section Pseudodysosmia (Passifloraceae). Syst Bot Monogr 41:1–446
Masters MT (1869) Vegetable teratology. Ray Society, London
Masters MT (1871) Contribution to the natural history of the Passifloraceae. Trans Linn Soc Lond Bot 27:593–645
Muschner VC, Lorenz AP, Cervi AC, Bonatto SL, Souza-Chies TT, Salzano FM, Freitas LB (2003) A first molecular phylogenetic analysis of Passiflora (Passifloraceae). Am J Bot 90:1229–1238
Payer J-B (1857) Traité d’organogénie comparée de la fleur. Victor Masson, Paris
Prenner G, Klitgaard BB (2008) Towards unlocking the deep nodes of Leguminosae: floral development and morphology of the enigmatic Duparquetia orchidacea (Leguminosae, Caesalpinioideae). Am J Bot 95:1349–1365
Prenner G, Deutsch G, Harvey P (2002) Floral developmet and morphology in Cuscuta reflexa Roxb. (Convolvulaceae). Stapfia 80:311–322
Prenner G, Vergara-Silva F, Rudall PJ (2009) The key role of morphology in modelling inflorescence architecture. Trends Plant Sci 14:302–309
Prenner G, Bateman RM, Rudall PJ (2010) Floral formulae updated for routine inclusion in formal taxonomic descriptions. Taxon 59:241–250
Puri V (1948) Studies in floral anatomy, V. On the structure and nature of the corona in certain species of the Passifloraceae. J Indian Bot Soc 27:130–149
Ronse De Craene LP (2010) Floral diagrams. Cambridge University Press, Cambridge
Sajo G, de Mello-Silva R, Rudall PJ (2010) Homologies of floral structures in Velloziaceae with particular reference to the corona. Int J Plant Sci 171:595–606
Sazima M, Buzato S, Sazima I (1999) Bat-pollinated flower assemblages and bat visitors at two Atlantic forest sites in Brazil. Ann Bot 83:705–712
Schleiden JM (1849) Principles of scientific botany. Longman, Brown, Green and Longmans, London
Schönenberger J, Grenhagen A (2005) Early floral development and androecium organization in Fouquieriaceae (Ericales). Plant Syst Evol 254:233–249
Schwabe WW, Clewer AG (1984) Phyllotaxis—a simple computer model based on the theory of a polarly-translocated inhibitor. J Theor Biol 109:595–619
Shah JJ, Dave YS (1970) Tendrils of Passiflora foetida: histogenesis and morphology. Am J Bot 57:786–793
Snow N, MacDougal JM (1993) New chromosome reports in Passiflora (Passifloraceae). Syst Bot 18:261–273
Thury M (1897) Observations sur la morphologie et l’organogénie florales des Passiflores. Bull Herb Boissier 5:494–503
Tokuoka T (2012) Molecular phylogenetic analysis of Passifloraceae sensu lato (Malpighiales) based on plastid and nuclear DNA sequences. J Plant Res 125:489–497
Traas J (2013) Phyllotaxis. Development 140:249–253
Vitta FA, Bernacci LC (2004) A new species of Passiflora in section Tetrastylis (Passifloraceae) and two overlooked species of Passiflora from Brazil. Brittonia 56:89–95
von Mohl H (1827) Über den Bau und das Winden der Ranken und Schlingpflanzen. Heinrich Laupp, Tübingen
Waters MT, Tiley AMM, Kramer EM, Meerow AW, Langdale JA, Scotland RW (2013) The corona of the daffodil Narcissus bulbocodium shares stamen-like identity and is distinct from the orthodox floral whorls. Plant J 74:615–625
Acknowledgments
Many thanks to John Longino (The Evergreen State College, Olympia, WA, USA) for identifying the captured ant to genus level (further determination not possible based on the evidence provided), Felicitas and Veronika Kollman (B-Rainforest, Costa Rica) for their generous hospitability during two visits to their finca, Paula Rudall and two anonymous reviewers for their valuable comments on the manuscript and the Office of International Relations (Karl-Franzens University Graz, Austria) for a travel grant to visit Costa Rica in 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prenner, G. Floral ontogeny in Passiflora lobata (Malpighiales, Passifloraceae) reveals a rare pattern in petal formation and provides new evidence for interpretation of the tendril and corona. Plant Syst Evol 300, 1285–1297 (2014). https://doi.org/10.1007/s00606-013-0961-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0961-0