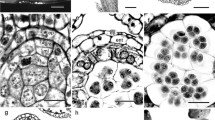
Abstract
Androecium development and vasculature were studied in nine species of the Adansonieae clade (core Bombacoideae, Malvaceae s.l.). In early androecium development either distinct pentagonal androecial ring walls or five common petal/androecium primordia are present. Ring walls give rise to five antepetalous and five alternipetalous primary androecial primordia. Common primordia divide into peripheral petal primordia and antepetalous primary androecial primordia. Antepetalous primary androecial primordia split anticlinally into ten primordia-halves, on which secondary androecial primordia are initiated in a centrifugal succession. Androecial lobes are formed by fusion of an alternipetalous primary androecial primordium and its two neighbouring antepetalous primary primordia-halves, a pattern that also occurs in other Malvatheca. Later, tertiary androecial primordia are formed by the subdivision of secondary androecial primordia (except in Adansonia and Ceiba). Each tertiary primordium differentiates into a two-locular androecial unit. At anthesis these two-locular androecial units are often present in pairs, corresponding to the two halves of the same secondary androecial primordium. Androecium development and vasculature imply that the alternipetalous androecial sectors have been reduced in Bombacoideae, a tendency that is shared with other subfamilies of Malvaceae.














Similar content being viewed by others
References
Aloni R (2001) Foliar and axial aspects of vascular differentiation—hypotheses and evidence. J Plant Growth Regul 20:22–34
Alverson WS, Karol KG, Baum DA, Chase MW, Swensen SM, McCourt R, Sytsma KJ (1998) Circumscription of the Malvales and relationships to other Rosidae: evidence from rbcL sequence data. Amer J Bot 85:876–887
Alverson WS, Whitlock BA, Nyffeler R, Bayer C, Baum DA (1999) Phylogeny of the core Malvales: evidence from ndhF sequence data. Amer J Bot 86:1474–1486
Arber A (1933) Floral anatomy and its morphological interpretation. New Phytol 32:231–242
Baum DA (1995) The comparative pollination and floral biology of baobabs (Adansonia-Bombacaceae). Ann Missouri Bot Gard 82:322–348
Baum DA, Small RL, Wendel JF (1998) Biogeography and floral evolution of Baobabs (Adansonia, Bombacaceae) as inferred from multiple data sets. Syst Biol 47:181–207
Baum DA, Smith SD, Yen A, Alverson WS, Nyffeler R, Whitlock BA, Oldham RL (2004) Phylogenetic relationships of Malvatheca (Bombacoideae and Malvoideae; Malvaceae sensu lato) as inferred from plastid DNA sequences. Amer J Bot 91:1862–1870
Bayer C, Kubitzki K (2003) Malvaceae. In: Kubitzki K, Bayer C (eds) The families and genera of vascular plants. Flowering plants, Dicotyledons, Malvales, Capparales and non-betalain Caryophyllales, vol 5. Springer, Berlin, pp 225–311
Bayer C, Fay MF, de Bruijn AY, Savolainen V, Morton CM, Kubitzki K, Alverson WS, Chase MW (1999) Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: a combined analysis of plastid atpB and rbcL DNA sequences. Bot J Linn Soc 129:267–303
Cheung M, Sattler R (1967) Early floral development of Lythrum salicaria. Canad J Bot 45:1609–1618
Coe MJ, Isaac FM (1965) Pollination of the baobab (Adansonia digitata L.) by the Lesser bush baby (Galago crassicaudatus E. Geoffroy). East Afr Wildl J 3:123–124
Davis TA (1966) Floral structure and stamens in Bombax ceiba. J Genet 59:294–328
Davis TA, Kundu A (1965) Floral structure and stamens in Ceiba pentandra (Linn.). Gaertn. J Bombay Nat Hist Soc 62:394–411
Davis TA, Mariamma KO (1965) The three kinds of stamens in Bombax ceiba L. Bull Jardin Bot État Bruxelles 35:185–211
Davis TA, Ghosh RB (1970) Comparative morphology of Bombax albidum and B.ceiba. Phytomorphology 20:339–351
Dengler NG (2001) Regulation of vascular development. J Plant Growth Regul 20:1–13
Duchartre MP (1845) Observations sur l′organogénie de la fleur dans les plantes de la famille des Malvacées. Ann Sci Nat Bot 4:123–150
Edlin HL (1935) A critical revision of certain taxonomic groups of the Malvales. New Phytol 34(1–20):122–143
Eichler AW (1875/1878) Blüthendiagramme. Engelmann, Leipzig
Endress PK, Stumpf S (1990) Non-tetrasporangiate stamens in the angiosperms: structure, systematic distribution and evolutionary aspects. Bot Jahrb Syst 112:193–240
Eguiarte L, Martinez del Rio C, Arita H (1987) El néctar y el pollen como recursos: El papel ecologico de los visitantes a las flores de Pseudobombax ellipticum (H·B.K.) Dugand. Biotropica 19:74–82
Elmqvist T, Cox PA, Rainey WE, Pierson ED (1992) Restricted pollination on Oceanic Islands: pollination of Ceiba pentandra by flying foxes in Samoa. Biotropica 24:15–23
Faegri K, van der Pijl L (1979) The priniciples of pollination ecology, 2nd edn. Pergamon Press, Oxford
Gribel R, Gibbs PE (2002) High outbreeding as a consequence of selfed ovule mortality and single vector bat pollination in the Amazonian tree Pseudobombax munguba (Bombacaceae). Int J Pl Sci 163:1035–1043
Gibbs PE, Semir J (2003) A taxonomic revision of the genus Ceiba MILL. (Bombacaceae). An Jard Bot Madr 60:259–300
Gibbs PE, Alverson WS (2006) How many species of Spirotheca (Malvaceae s.l., Bombacoideae)? Brittonia 58(3):245–258
Gibbs PE, Semir J, Da Cruz ND (1988) A proposal to unite the genera Chorisia Knuth with Ceiba Miller (Bombacaceae). Notes Roy Bot Gard Edinburgh 45:125–136
Gribel R, Gibbs PE, Queiroz AI (1999) Flowering phenology and pollination biology of Ceiba pentandra (Bombacaceae) in Central Amazonia. J Trop Ecol 15:247–263
Grünmeier R (1999) Fledermausblumen und ihre Bestäuber. Palmengarten-Sonderheft 31:95–106
Hirmer M (1918) Beiträge zur Morphologie der polyandrischen Blüten. Flora 110:140–192
Hufford L (1998) Early development of androecia in polystemonous Hydrangeaceae. Amer J Bot 85(9):1057–1067
Hutchinson J (1967) The genera of flowering plants, Dicotyledones, vol 2. Clarendon Press, Oxford
Igersheim A (1993) The character states of the Caribbean monotypic Strumpfia (Rubiaceae). Nord J Bot 13:545–559
Igersheim A, Cichocki O (1996) A simple method for microtome sectioning of prehistoric charcoal specimens embedded in 2-hydroxyethyl methacrylate (HEMA). Rev Palaeobot Palynol 92:389–393
Jaeger P (1974) Contribution à l′étude de la chiroptèrogamie. Le Balsa (Ochroma lagopus Sw., Bombacacées). Bull Inst Fond Afr Noire A 36:886–899
Janka H (2003) Floral ontogeny in Malvaceae-Malvoideae. Biodivers Evol Biol: 175. Abstract
Jenny M (1989) Organstellung und Androeceumentwicklung ausgewählter Sterculiaceae. In: 9th Symp. Morph Anat Syst, Wien 26
Kaisila J (1966) The Egyptian fruit-bat, Rousettus aegyptiacus Geoffr. (Megachiroptera, Pteropodidae) visiting flowers of Bombax malabaricum. Ann Zool Fenn 3:1–3
Leins P (1975) Die Beziehungen zwischen multistaminaten und einfachen Androeceen. Bot Jahrb Syst 96:231–237
Lobo JA, Quesada M, Stoner KE (2005) Effects of pollination by bats on the mating system of Ceiba pentandra (Bombacaceae) populations in two tropical life zones in Costa Rica. Amer J Bot 92:370–376
MacFarlane AT, Mori SA, Purzycki K (2003) Notes on Eriotheca longitubulosa (Bombacaceae), a rare, putatively hawkmoth-pollinated species new to the Guianas. Brittonia 55:305–316
Nandi OI (1998) Floral development and systematics of Cistaceae. Pl Syst Evol 212:107–134
Neubauer HF (1964) Die Dschungelkrähe, Corvus macrolynchus Wagler als Bestäuber der Blüten von Bombax malabricum DC. Ber Dtsch Bot Ges 77:219–223
Neubauer HF (1989) Bemerkungen über das Androecium von Bombax ceiba L. (Bombacaceae). Oberhess. Naturwiss Z 51:99–105
Niinemets Ü, Reichstein M, Staudt M, Seufert G, Tenhunen JD (2002) Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Pl Physiol 130:1371–1385
Nyffeler R, Bayer C, Alverson WS, Yen A, Whitlock BA, Chase MW, Baum DA (2005) Phylogenetic analysis of the Malvadendrina clade (Malvaceae s.l.) based on plastid DNA sequences. Org Divers Evol 5:109–125
Oliveira PE, Gibbs PE, Barbosa AA, Talavera S (1992) Contrasting breeding systems in two Eriotheca (Bombacaceae) species of the Brazilian cerrados. Pl Syst Evol 179:207–219
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Timber Press, Portland, Oregon
Rao VC (1952) Floral anatomy of some Malvales and its bearing on the affinities of families included in the order. J Indian Bot Soc 31:171–203
Reddi CS, Jyothi PV, Atluri JB (1989) Reproductive ecology of silk cotton tree, Ceiba pentandra L., Indian J Palynol 25:93–105
Robyns A (1963) Essai de monographie du genre Bombax s.l. (Bombacaceae). Bull Jard Bot Etat Bruxelles 33:1–316
Rohweder O (1972) Das Andröcium der Malvales und der ‘Konservatismus’ des Leitgewebes. Bot Jahrb Syst 92:155–167
Ronse Decraene LP (1988) Two types of ringwall formation in the development of complex polyandry. Bull Soc Roy Belg 121:122–124
Ronse Decraene LP (1989) Floral development of Cochlospermum tinctorium and Bixa orellana with special emphasis on the androecium. Amer J Bot 76:1344–1359
Ronse Decraene LP, Smets EF (1992) Complex polyandry in the Magnoliatae: definition, distribution and systematic value. Nord J Bot 12:621–649
Ronse Decraene LP, Clinckemaillie D, Smets EF (1993) Stamen-petal complexes in Magnoliatae. Bull Jard Bot Nat Belg 62:97–112
Ronse Decraene LP, Smets EF (1993) Dédoublement revisited: towards a renewed interpretation of the androecium of the Magnoliophytina. Bot J Linn Soc 113:103–124
Ronse Decraene LP, Smets EF (1995) The distribution and systematic relevance of the androecial character oligomery. Bot J Linn Soc 118:193–247
Ronse Decraene LP, Smets EF (1996) The morphological variation and systematic value of stamen pairs in the Magnoliatae. Feddes Repert 107:1–17
Sattler R (1973) Organogenesis of flowers. University of Toronto Press, Toronto
Saunders ER (1937) Floral morphology, vol I. Heffer, London
Schumann KM (1890) Bombacaceae. In: Engler A, Prantl K (eds) Die Natürlichen Pflanzenfamilien 3(6): 53–68. Leipzig
Takhtajan A (1997) Diversity and classification of flowering plants. Columbia University Press, New York
van der Pijl L (1936) Fledermäuse und Blumen. Flora 131:1–40
van Heel WA (1966) Morphology of the androecium in Malvales. Blumea 13:177–349
van Heel WA (1969) The synangial nature of pollen sacs on the strength of ‘congenital fusion’ and conservation of the vascular bundle system, with special reference to some Malvales I, II. Koninkl Nederl Akad Wet 72:172–206
Vogel S (1969) Chiropterophilie in der neotropischen Flora. Flora 158:185–222
von Balthazar M, Nyffeler R (2002) The peculiar androecium of Cullenia (Durioneae, Malvaceae s.l.). In: Schönenberger J, von Balthazar M, Matthews M (eds) Flowers: diversity, development & evolution. Institute of Systematic Botany, University of Zurich, Switzerland
von Balthazar M, Alverson WS, Schönenberger J, Baum DA (2004) Comparative floral development and androecium structure in Malvoideae (Malvaceae s.l.). Int J Pl Sci 165:445–473
von Balthazar M, Schönenberger J, Alverson WS, Janka H, Bayer C, Baum DA (2006) Structure and evolution of the androecium in the Malvatheca clade (Malvaceae s.l.) and implications for Malvaceae and Malvales. Pl Syst Evol 260:171–197
Wilson CL (1937) The phylogeny of the stamen. Amer J Bot 24:686–699
Acknowledgments
For valuable fixed plant material we would like to thank W. S. van Heel; L. Y. S. Aona; Sabah Agriculture Park, Tenom, Malaysia; Olbrich Botanic Gardens, Madison, WI, USA; Fairchild Tropical Garden, Coral Gables, FL, USA; Botanic Garden of the University of Zurich, Zurich, Switzerland and Palmengarten, Frankfurt am Main, Germany. We acknowledge the Laboratory of the Sect. Marine Evertebrates II, Senckenberg Research Institute in Frankfurt am Main and the BBPIC Laboratory of the University of Wisconsin, Madison for the use of the scanning electron microscopes. We thank Georg Zizka, Senckenberg Research Institute, Frankfurt am Main, and Matthias Jenny, Palmengarten, Frankfurt am Main for enabling access to laboratory and SEM. We are very grateful to Dieter Fiege and Marie Louise Tritz, Senckenberg Research Institute, Frankfurt am Main, for valuable aid and assistance on the SEM. For valuable comments on the manuscript we thank Ulrike Brunken. M. von Balthazar received financial support by grants from the National Science Foundation (NSF) to D. A. Baum (DEB 9876070; 0416096) and to W. S. Alverson (BSF-8800193) and by a postdoctoral fellowship from the Forschungskommission of the University of Zurich, Switzerland, and from the Swedish Research Council to E. M. Friis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janka, H., von Balthazar, M., Alverson, W.S. et al. Structure, development and evolution of the androecium in Adansonieae (core Bombacoideae, Malvaceae s.l.). Plant Syst Evol 275, 69–91 (2008). https://doi.org/10.1007/s00606-008-0055-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0055-6