Abstract
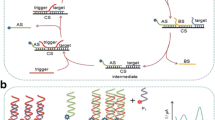
The authors describe a label-free electrochemical aptasensor for ultrasensitive and highly specific detection of the antibiotic based on cascade enzymatic recycling couple with DNAzyme amplification. The assay involves two sequential reactions: the first reaction is a λ exonuclease-assisted cyclic digestion reaction triggered by target-aptamer binding. The second reaction is a nicking endonuclease-aided cyclic nicking reaction, which produces a large amount of G-rich nucleic acid segments. These form a G-quadruplex/hemin complex in the presence of K(I) ions and hemin. Because the G-quadruplex/hemin complex acts as a horseradish peroxidase-mimicking DNAzyme with excellent redox activity, the electrochemical signal transduction is accomplished due to the electroreduction of H2O2. It appears that this work is the first example that cascade enzymatic recycling coupled to DNAzyme amplification is used for antibiotic detection. The aptasensor was applied to the quantitation of kanamycin and gave a response that is linear in the 1 pM to 10 nM kanamycin concentration range, with a detection limit as low as 0.5 pM. The working voltage (vs. Ag/AgCl) at which data can be acquired best is −0.35 V. The assay offers the advantages of remarkably improved sensitivity, use of affordable instrumentation, and simplified operation without the need for electrochemical labeling or addition of labile reagents. Thus, cascade enzymatic recycling coupled to DNAzyme amplification represents a versatile platform for highly sensitive and specific antibiotics detection.

ᅟ




Similar content being viewed by others
References
Zhou LJ, Ying GG, Zhao JL, Yang JF, Wang L, Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Conzuelo F, Gamella M, Campuzano S, Pinacho DG, Reviejo AJ, Marco MP, Pingarrón JM (2012) Disposable and integrated amperometric immunosensor for direct determination of sulfonamide antibiotics in milk. Biosens Bioelectron 36:81–88
Megoulas NC, Koupparis MA (2004) Direct determination of kanamycin in raw materials, veterinary formulation and culture media using a novel liquid chromatography-evaporative light scattering method. Anal Chim Acta 547:64–72
Huang YJ, Cheng MM, Li WH, Wu LH, Chen YS, Luo YM, Christie P, Zhang HB (2013) Simultaneous extraction of four classes of antibiotics in soil, manure and sewage sludge and analysis by liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal Methods 5:3721–3731
Sierra-Rodero M, Fernández-Romero JM, Gómez-Hens A (2014) Determination of fluoroquinolone antibiotics by microchip capillary electrophoresis along with time-resolved sensitized luminescence of their terbium(III) complexes. Microchim Acta 181:1897–1904
Mariani S, Ermini ML, Scarano S, Bellissima F, Bonini M, Berti D, Minunni M (2013) Improving surface plasmon resonance imaging of DNA by creating new gold and silver based surface nanostructures. Microchim Acta 180:1093–1099
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA that bind specific ligands. Nature 346:818–822
Wu SJ, Li Q, Duan N, Ma HL, Wang ZP (2016) DNA aptamer selection and aptamer-based fluorometric displacement assay for the hepatotoxin microcystin-RR. Microchim Acta 183:2555–2562
Tang DP, Hou L (2016) Aptasensor for ATP based on analyte-induced dissociation of ferrocene-aptamer conjugates from manganese dioxide nanosheets on a screen-printed carbon electrode. Microchim Acta 183:2705–2711
Dai H, Zhang SP, Hong ZS, Lin YY (2016) A potentiometric addressable Photoelectrochemical biosensor for sensitive detection of two biomarkers. Anal Chem 88:9532–9538
Kurita R, Arai K, Nakamoto K, Kato D, Niwa O (2012) Determination of DNA methylation using Electrochemiluminescence with surface Accumulable Coreactant. Anal Chem 84:1799–1803
Wang YS, Ma TC, Ma SY, Liu YJ, Tian YP, Wang RN, Jiang YB, Hou DJ, Wang JL (2017) Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim Acta 184:203–210
Wang MH, Zhang S, Ye ZH, Peng DL, He LH, Yan FF, Yang YQ, Zhang HZ, Zhang ZH (2015) A gold electrode modified with amino-modified reduced graphene oxide, ion specific DNA and DNAzyme for dual electrochemical determination of Pb(II) and hg(II). Microchim Acta 182:2251–2258
Wang HZ, Wang Y, Liu S, Yu JH, Xu W, Guo YN, Huang JD (2015) Target–aptamer binding triggered quadratic recycling amplification for highly specific and ultrasensitive detection of antibiotics at the attomole level. Chem Commun 51:8377–8380
Cui HF, Xu TB, Sun YL, Zhou AW, Cui YH, Liu W, Luong HTJ (2015) Hairpin DNA as a Biobarcode modified on gold nanoparticles for electrochemical DNA detection. Anal Chem 87:1358–1365
Zhu XL, Zhang HH, Feng C, Ye ZH, Li GX (2014) A dual-colorimetric signal strategy for DNA detection based on graphene and DNAzyme. RSC Adv 4:2421–2426
Liu SF, Gong HW, Wang YQ, Wang L (2016) Label-free electrochemical nucleic acid biosensing by tandem polymerization and cleavage-mediated cascade target recycling and DNAzyme amplification. Biosens Bioelectron 77:818–823
Zhou WJ, Gong X, Xiang Y, Yuan R, Chai YQ (2014) Target-triggered quadratic amplification for label-free and sensitive visual detection of cytokines based on hairpin aptamer DNAzyme probes. Anal Chem 86:953–958
Xu Y, Wang Y, Liu S, Yu JH, Wang HZ, Guo YN, Huang JD (2016) Ultrasensitive and rapid detection of miRNA with three-way junction structure-based trigger-assisted exponential enzymatic amplification. Biosens Bioelectron 81:236–241
Zhao HZ, Dong JJ, Zhou FL, Li BX (2015) G-quadruplex-based homogenous fluorescence platform for ultrasensitive DNA detection through isothermal cycling and cascade signal amplification. Microchim Acta 182:2495–2502
Meng Y, Hun X, Zhang Y, Luo X (2016) Toehold-aided DNA recycling amplification using hemin and G-quadruplex reporter DNA on magnetic beads as tags for chemiluminescent determination of riboflavin. Microchim Acta 183:2965–2971
He Y, Jiao B (2016) Simple and convenient G-quadruplex-based fluorescent assay of biotin-streptavidin interaction via terminal protection of small molecule-linked DNA. Microchim Acta 183:3303–3309
Yang J, Xiang Y, Song C (2015) Quadruple signal amplification strategy based on hybridization chain reaction and an immunoelectrode modified with graphene sheets, a hemin/G-quadruplex DNAzyme concatamer, and alcohol dehydrogenase: ultrasensitive determination of influenza virus subtype H7N9. Microchim Acta 182:2377–2385
Travascio P, Witting PK, Mauk AG, Sen D (2001) The peroxidase activity of a hemin -DNA oligonucleotide complex: free radical damage to specific guanine bases of the DNA. J Am Chem Soc 123:1337–1248
Li T, Shi LL, Wang EK, Dong SJ (2009) Multifunctional G-Quadruplex aptamers and their application to protein detection. Chem Eur J 15:1036–1042
Loomans E, Wiltenburg J, Koets M, Amerongen AV (2003) Neamin as an immunogen for the development of a generic ELISA detecting gentamicin, kanamycin, and neomycin in milk. J Agric Food Chem 51:587–593
Song KM, Cho M, Jo H, Min K, Jeon SH, Kim T, Han MS, Ku JK, Ban C (2011) Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem 415:175–181
Yu SJ, Wei Q, Du B, Wu D, Li H, Yan LG, Ma HM, Zhang Y (2013) Label-free immunosensor for the detection of kanamycin using ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Anal Biochem 415:175–181
Wang XY, Zou MJ, Xu X, Lei R, Li K, Li N (2009) Determination of human urinary kanamycin in one step using urea-enhanced surface plasmon resonance light-scattering of gold nanoparticles. Anal Bioanal Chem 395:2397–2403
Li RZ, Liu Y, Cheng L, Yang CZ, Zhang JD (2014) Photoelectrochemical Aptasensing of kanamycin using visible LightActivated carbon nitride and graphene oxide nanocomposites. Anal Chem 86:9372–9375
Liao QG, Wei BH, Luo LG (2017) Aptamer based fluorometric determination of kanamycin using double-stranded DNA and carbon nanotubes. Microchim Acta 184:627–632
Liu C, Lu C, Tang Z, Chen X, Wang G, Sun F (2015) Aptamer-functionalized magnetic nanoparticles for simultaneous fluorometric determination of oxytetracycline and kanamycin. Microchim Acta 182:2567–2575
He JX, Wang Y, Zhang XY (2016) Preparation of artificial antigen and development of IgY-based indirect competitive ELISA for the detection. Microchim Acta 9:744–751
Wang CK, Wang CG, Wang QQ, Chen D (2017) Resonance light scattering method for detecting kanamycin in milk with enhanced sensitivity. Anal Bioanal Chem 409:2839–2846
Acknowledgements
This work was supported by NSFC (21405060, 1471644), Shandong Province Natural Science Funds for Distinguished Young Scholars (JQ201410), and Shandong Province Natural Science Funds (ZR2015CM027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 4.82 mb)
Rights and permissions
About this article
Cite this article
Han, C., Li, R., Li, H. et al. Ultrasensitive voltammetric determination of kanamycin using a target-triggered cascade enzymatic recycling couple along with DNAzyme amplification. Microchim Acta 184, 2941–2948 (2017). https://doi.org/10.1007/s00604-017-2311-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2311-3