Abstracts
The authors describe a dual-signal electrochemical biosensor for highly sensitive determination of the HIV-1 related gene. This method is based on the application of cascaded toehold-mediated strand displacement reactions (TMSDRs) in combination with non-enzymatic target recycling amplification (TRA). A DNA machine with two TMSDRs was designed, and this resulted in reusable target and an output of two oligonucleotides, referred to as strand A (AS) labeled with the redox tag methylene blue (MB) and as untagged strand B (BS). A ferrocene (Fc)-modified signal probe (Fc-P1) is immobilized on the gold electrode surface by hybridizing with a thiolated probe (P2). The labeled AS causes the dissociation of Fc molecules and the gathering of MB molecules via strand displacement reaction. The target gene triggers TMSDRs and TRA. This leads to an increase in the distance changes between the redox tags and the gold electrode. The assay works in the 1 pM to 10 nM concentration range. On account of target recycling and dual recognition, the limit of detection is as low as 0.88 pM (at an S/N ratio of 3). The assay also has a remarkable selectivity which is ascribed to the use of both cascaded TMSDRs and dual recognition. In our perception, this assay represents a robust means of wide scope in that it may be applied to the detection of various kinds of nucleic acid even in complex samples.

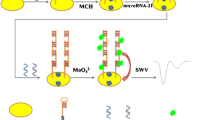
Schematic of a dual-signal electrochemical biosensor based on cascaded toehold-mediated strand displacement and non-enzymatic target recycling amplification strategy for the HIV-1 related gene detection. Two electroactive molecules are used to produce electrochemical signals in this “signal-on/off” sensing system.





Similar content being viewed by others
References
World Health Organization (2015) Global health sector response to HIV, 2000–2015: focus on innovations in Africa: progress report
Holmström P, Syrjänen S, Laine P, Valle SL, Suni J (1990) HIV antibodies in whole saliva detected by ELISA and Western blot assays. J Med Virol 30(4):245–248
Roth WK, Weber M, Seifried E (1999) Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus, and HIV-1 in a blood-bank setting. Lancet 353(9150):359–363
Yarchoan R, Mitsuya H, Broder S (1993) Challenges in the therapy of HIV infection. Immunol Today 14(6):303–309
Meng Q, Wong C, Rangachari A, Tamatsukuri S, Sasaki M, Fiss E, Cheng L, Ramankutty T, Clarke D, Yawata H (2001) Automated multiplex assay system for simultaneous detection of hepatitis B virus DNA, hepatitis C virus RNA, and human immunodeficiency virus type 1 RNA. J Clin Microbiol 39(8):2937–2945
Edelmann A, Kalus U, Oltmann A, Stein A, Unbehaun A, Drosten C, Krüger DH, Hofmann J (2010) Improvement of an ultrasensitive human immunodeficiency virus type 1 real-time reverse transcriptase-polymerase chain reaction targeting the long terminal repeat region. Transfusion 50(3):685–692
Kiselinova M, Pasternak AO, De Spiegelaere W, Vogelaers D, Berkhout B, Vandekerckhove L (2014) Comparison of droplet digital PCR and seminested real-time PCR for quantification of cell-associated HIV-1 RNA. PLoS One 9(1):e85999
Mercier-Delarue S, Vray M, Plantier JC, Maillard T, Adjout Z, de Olivera F, Schnepf N, Maylin S, Simon F, Delaugerre C (2014) Higher specificity of nucleic acid sequence-based amplification isothermal technology than of real-time PCR for quantification of HIV-1 RNA on dried blood spots. J Clin Microbiol 52(1):52–56
Linnen JM, Gilker JM, Menez A, Vaughn A, Broulik A, Dockter J, Gillotte-Taylor K, Greenbaum K, Kolk DP, Mimms LT (2002) Sensitive detection of genetic variants of HIV-1 and HCV with an HIV-1/HCV assay based on transcription-mediated amplification. J Virol Methods 102(1):139–155
Clark LC, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102(1):29–45
Pan L-H, Kuo S-H, Lin T-Y, Lin C-W, Fang P-Y, Yang H-W (2017) An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosens Bioelectron 89:598–605
Zhang X, Zhou D, Sheng S, Yang J, Chen X, Xie G, Xiang H (2016) Electrochemical immunoassay for the cancer marker LMP-1 (Epstein-Barr virus-derived latent membrane protein 1) using a glassy carbon electrode modified with Pd@ Pt nanoparticles and a nanocomposite consisting of graphene sheets and MWCNTs. Microchim Acta 183:2055–2062
Lin M, Song P, Zhou G, Zuo X, Aldalbahi A, Lou X, Shi J, Fan C (2016) Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat Protoc 11(7):1244–1263
Rasheed PA, Sandhyarani N (2017) Electrochemical DNA sensors based on the use of gold nanoparticles: a review on recent developments. Microchim Acta 184:981–1000
Li J, Chen Z, Xiang Y, Zhou L, Wang T, Zhang Z, Sun K, Yin D, Li Y, Xie G (2016) An electrochemical biosensor for double-stranded Wnt7B gene detection based on enzymatic isothermal amplification. Biosens Bioelectron 86:75–82
Moon J, Ha Y, Kim M, Sim J, Lee Y, Suh M (2016) Dual electrochemical Microsensor for real-time simultaneous monitoring of nitric oxide and potassium ion changes in a rat brain during spontaneous neocortical epileptic seizure. Anal Chem 88(18):8942–8948
Sepunaru L, Sokolov SV, Holter J, Young NP, Compton RG (2016) Electrochemical red blood cell counting: one at a time. Angew Chem Int Ed 128(33):9920–9923
Cheng W, Zhang W, Yan Y, Shen B, Zhu D, Lei P, Ding S (2014) A novel electrochemical biosensor for ultrasensitive and specific detection of DNA based on molecular beacon mediated circular strand displacement and rolling circle amplification. Biosens Bioelectron 62:274–279
Wang Q, Yang C, Xiang Y, Yuan R, Chai Y (2014) Dual amplified and ultrasensitive electrochemical detection of mutant DNA biomarkers based on nuclease-assisted target recycling and rolling circle amplifications. Biosens Bioelectron 55:266–271
Xie S, Yuan Y, Chai Y, Yuan R (2015) Tracing phosphate ions generated during loop-mediated isothermal amplification for electrochemical detection of Nosema Bombycis genomic DNA PTP1. Anal Chem 87(20):10268–10274
Barreda-García S, González-Álvarez MJ, Palacios-Gutiérrez JJ, Miranda-Ordieres AJ, Lobo-Castañón MJ (2015) Attomolar quantitation of mycobacterium tuberculosis by asymmetric helicase-dependent isothermal DNA-amplification and electrochemical detection. Biosens Bioelectron 68:122–128
Wang Q, Yang L, Yang X, Wang K, He L, Zhu J, Su T (2012) An electrochemical DNA biosensor based on the “Y” junction structure and restriction endonuclease-aided target recycling strategy. Chem Commun 48(24):2982–2984
Yurke B, Turberfield AJ, Mills AP, Simmel FC, Neumann JL (2000) A DNA-fuelled molecular machine made of DNA. Nature 406(6796):605–608
Zhang DY, Winfree E (2009) Control of DNA strand displacement kinetics using toehold exchange. J Am Chem Soc 131(47):17303–17314
Zhang DY, Turberfield AJ, Yurke B, Winfree E (2007) Engineering entropy-driven reactions and networks catalyzed by DNA. Science 318(5853):1121–1125
Chen HG, Ren W, Jia J, Feng J, Gao ZF, Li NB, Luo HQ (2016) Fluorometric detection of mutant DNA oligonucleotide based on toehold strand displacement-driving target recycling strategy and exonuclease III-assisted suppression. Biosens Bioelectron 77:40–45
Qian L, Winfree E (2011) Scaling up digital circuit computation with DNA strand displacement cascades. Science 332(6034):1196–1201
Engelen W, Meijer LH, Somers B, Merkx M (2017) Antibody-controlled actuation of DNA-based molecular circuits. Nat Commun 8:14473
Peng P, Shi L, Wang H, Li T (2016) A DNA nanoswitch-controlled reversible nanosensor. Nucleic Acids Res 45:541–546
Wang T, Zhou L, Bai S, Zhang Z, Li J, Jing X, Xie G (2016) Ultraspecific electrochemical DNA biosensor by coupling spontaneous cascade DNA branch migration and dual-signaling sensing strategy. Biosens Bioelectron 78:464–470
Wang L, Tian J, Huang Y, Lin X, Yang W, Zhao Y, Zhao S (2016) Homogenous fluorescence polarization assay for the DNA of HIV a T7 by exploiting exonuclease-assisted quadratic recycling amplification and the strong interaction between graphene oxide and ssDNA. Microchim Acta 183(7):2147–2153
Tran LD, Nguyen BH, Van Hieu N, Tran HV, Nguyen HL, Nguyen PX (2011) Electrochemical detection of short HIV sequences on chitosan/Fe3O4 nanoparticle based screen printed electrodes. Mater Sci Eng C 31(2):477–485
Li B, Li Z, Situ B, Dai Z, Liu Q, Wang Q, Gu D, Zheng L (2014) Sensitive HIV-1 detection in a homogeneous solution based on an electrochemical molecular beacon coupled with a nafion-graphene composite film modified screen-printed carbon electrode. Biosens Bioelectron 52:330–336
Wang X, Jiang A, Hou T, Li F (2015) A versatile label-free and signal-on electrochemical biosensing platform based on triplex-forming oligonucleotide probe. Anal Chim Acta 890:91–97
Huang J, Su X, Li Z (2012) Enzyme-free and amplified fluorescence DNA detection using bimolecular beacons. Anal Chem 84:5939–5943
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 81672112) and Chongqing Yuzhong District Science and Technology Project (20140108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1148 kb)
Rights and permissions
About this article
Cite this article
Yin, D., Tao, Y., Tang, L. et al. Cascade toehold-mediated strand displacement along with non-enzymatic target recycling amplification for the electrochemical determination of the HIV-1 related gene. Microchim Acta 184, 3721–3728 (2017). https://doi.org/10.1007/s00604-017-2368-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2368-z