Abstract
The authors describe an ultra-sensitive approach for the determination of lead(II) by using a lead-specific aptamer and a fluorophore derived from perylenetetracarboxylic acid diimide (PTCDI). The fluorescence of PTCDI, with excitation/emission peaks at 494/543 nm, is quenched by the aptamer but restored if the aptamer binds lead(II). A detection scheme has been worked out that has a 0.1 ng·mL−1limit of detection and a dynamic range that extends from 0.1 ng·mL−1 to 10 μg·mL−1. The assay also demonstrates high selectivity for lead ions over nine other ions due to the aptamer’s high specificity. The method shows a average recovery of 77.2–93.4% with an RSD of less than 3% in water samples.

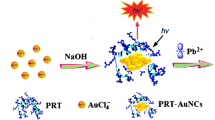
Schematic of an aptamer and perylenetetracarboxylic acid diimide (PTCDI) based fluorescent assay for lead (II). It has a dynamic range of 0.1 ng•mL−1 to 10 μg•mL−1 and a detection limit of 0.1 ng•mL−1.



Similar content being viewed by others
References
Sumontha N, Nuchanart R, Norratouch P, Jutamaad S (2016) Concentrations of trace elements in organic fertilizers and animal manures and feeds and cadmium contamination in herbal tea. J Agric Food Chem 64:3119–3126
Lu Y (2002) New transition-metal-dependent DNAzymes as efficient endonucleases and as selective metal biosensors. Chemistry 8:4589–4596
Needleman HL, Landrigan PJ (1981) The health effects of low level exposure to lead. Annu Rev Public Health 2:277–298
Needleman HL, Bellinger D (1991) The health effects of low level exposure to lead. Annu Rev Public Health 12:111–140
Ebdon L, Hill SJ, Rivas C (1998) Lead speciation in rainwater by isotope dilution-high performance liquid chromatography inductively coupled plasma mass spectrometry. Spectrochim Acta B 53:289–297
Szpunar J, Pellerin P, Makarov A, Doco T, Williams P, Medina B (1998) Speciation analysis for biomolecular complexes of lead in wine by size-exclusion high-performance liquid chromatography inductively coupled plasma mass spectrometry. J Anal Atom Spectrom 13:749–754
Hsieh HF, Chang WS, Hsieh YK, Wang CF (2009) Lead determination in whole blood by laser ablation coupled with inductively coupled plasma mass spectrometry. Talanta 79:183–188
Petrov PK, Wibetoe G, Tsalev DL (2006) Comparison between hydride generation and nebulization for sample introduction in the determination of lead in plants and water samples by inductively coupled plasma mass spectrometry, using external calibration and isotope dilution. Spectrochim Acta B 61:50–57
Goubert-Renaudin S, Moreau M, Despas C, Meyer M, Denat F, Lebeau B (2009) Voltammetric detection of lead(II) using amide-Cyclam-functionalized silica-modified carbon paste electrodes. Electroanalysis 21:1731–1742
Liu J, Guan Z, Lv Z, Jiang X, Yang S, Chen A (2014) Improving sensitivity of gold nanoparticle based fluorescence quenching and colorimetric aptasensor by using water resuspended gold nanoparticle. Biosens Bioelectron 52:265–270
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Munoz RAA, Silva CS, Correia PRM, Oliveira PV, Angnes L (2005) Potentiometric stripping analysis for simultaneous determination of copper and lead in lubricating oils after total digestion in a focused microwave-assisted oven. Microchim Acta 149:199–204
Baralkiewicz D, Kózka M, Gramowska H, Tomaszewska B, Wasinkiewicz K (2004) Determination of lead in plants in controlling phytoremediation processes using slurry sampling electrothermal atomic absorption spectrometry. Inter J Environ An Ch 84:901–908
Zhao M, Zhuo Y, Chai YQ, Xiang Y, Liao N, Gui GF (2013) Dual signal amplification strategy for the fabrication of an ultrasensitive electrochemiluminescenct aptasensor. Analyst 138:6639–6644
Mayer G (2009) The chemical biology of aptamers. Angew Chem 48:2672–2689
Zhu C, Zhang G, Huang Y, Yan J, Chen A (2017) Aptamer based ultrasensitive determination of the β-adrenergic agonist ractopamine using PicoGreen as a fluorescent DNA probe. Microchim Acta 184:439–444
Cerchia L, Giangrande PH, McNamara JO, De FV (2009) Cell-specific aptamers for targeted therapies. Methods Mol Biol 535:59–78
Lv ZZ, Liu J, Zhou Y, Guan Z, Yang S, Li C, Chen A (2013) Highly sensitive fluorescent detection of small molecules, ions, and proteins using a universal label-free aptasensor. Chem Comm 49:5465–5467
Qian ZS, Shan XY, Chai LJ, Chen JR, Peng H (2015) A fluorescent nanosensor based on graphene quantum dots-aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens Bioelectron 68:225–231
Taghdisi SM, Emrani SS, Tabrizian K, Ramezani M, Abnous K, Emrani AS (2014) Ultrasensitive detection of lead (II) based on fluorescent aptamer-functionalized carbon nanotubes. Environ Toxicol Phar 37:1236–1242
Li T, Dong SJ, Wang EK (2010) A lead(II)-driven DNA molecular device for turn-on fluorescence detection of lead(II) ion with high selectivity and sensitivity. J Am Chem Soc 132:13156–13157
Hai H, Yang F, Li J (2014) Highly sensitive electrochemiluminescence “turn-on” aptamer sensor for lead (II) ion based on the formation of a G-quadruplex on a graphene and gold nanoparticles modified electrode. Microchim Acta 181(9–10):893–901
Huang Y, Liu X, Zhang L, Hu K, Zhao S, Fang B (2015) Nicking enzyme and graphene oxide-based dual signal amplification for ultrasensitive aptamer-based fluorescence polarization assays. Biosens Bioelectron 63:178–184
Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R (2007) Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett 7:3065–3070
Hu PP, Zheng LL, Zhan L, Li JY, Zhen SJ, Liu H (2013) Metal-enhanced fluorescence of nano-core-shell structure used for sensitive detection of prion protein with a dual-aptamer strategy. Anal Chim Acta 787:239–245
Huang YF, Yan J, Chen AL, Zhang C, Bai WH (2016) Highly sensitive and selective optical detection of lead (II) using a label-free fluorescent Aptasensor. RSC Advance 6:90300–90304
Ju E, Yang X, Lin Y, Pu F, Ren J, Qu X (2012) Exonuclease-aided amplification for label-free and fluorescence turn-on DNA detection based on aggregation-induced quenching. Chem comm 48:11662–11664
Lv Z, Liu J, Bai W, Yang S, Chen A (2015) A simple and sensitive label-free fluorescent approach for protein detection based on a Perylene probe and aptamer. Biosens Bioelectron 64:530–534
Lv ZZ, Chen AL, Liu JC, Guan Z, Zhou Y, Xu SY (2014) A simple and sensitive approach for Ochratoxin a detection using a label-free fluorescent Aptasensor. PLoS One 9:e85968
Wang B, Yu C (2010) Fluorescence turn-on detection of a protein through the reduced aggregation of a Perylene probe. Angew Chem Int Edit 49:1485–1488
Kotch FW, JCF, Davis JT (2000) A lead-filled G-Quadruplex: insight into the G-Quartet’s selectivity for Pb2+ over K+. Org Lett 2:3277–3280
Zhan SS, Wu YG, Luo YF, Liu L, He L, Xing HB (2014) Label-free fluorescent sensor for lead ions detection based on lead(II)-stabilized G-quadruplex formation. Anal Biochem 462:19–25
Guo LQ, Nie DD, Qiu CY, Zheng QS, Wu HY, Ye PR (2012) A G-quadruplex based label-free fluorescent biosensor for lead ions. Biosens Bioelectron 35:123–127
Wang CX, Cheng H, Sun YQ, Xu ZZ, Lin HH, Lin Q (2015) Nanoclusters prepared from a silver/gold alloy as a fluorescent probe for selective and sensitive determination of lead(II). Microchim Acta 182:695–701
Qi YX, Zhang M, Fu QQ, Liu R, Shi GY (2013) Highly sensitive and selective fluorescent detection of cerebral lead(II) based on graphene quantum dot conjugates. Chem comm 49:10599–10601
Wang HY, Song ZY, Zhang HS, Chen SP (2016) Single-molecule analysis of lead(II)-binding aptamer conformational changes in an α-hemolysin nanopore and sensitive detection of lead(II). Microchim Acta 183:1003–1010
Xu H, Liu BX, Chen Y (2012) A colorimetric method for the determination of lead(II) ions using gold nanoparticles and a guanine-rich oligonucleotide. Microchim Acta 177:89–94
Fan CH, He SJ, Liu G, Wang LH, Song SP (2012) A portable and power-free microfluidic device for rapid and sensitive lead (Pb2+) detection. Sensors 12:9467–9475
Wang ZD, Lee JH, Lu Y (2008) Label-free colorimetric detection of lead ions with a nanomolar detection limit and tunable dynamic range by using gold nanoparticles and DNAzyme. Adv Mater 20:3263–3267
Acknowledgements
This work was supported by the Special Fund for Agro Scientific Research in the Public Interest (201203046, 201203023). The authors expressed their gratitude for the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 30.4 kb)
Rights and permissions
About this article
Cite this article
Yan, M., Zhu, C., Huang, Y. et al. Ultrasensitive detection of lead(II) using a turn-on probe based on the use of an aptamer and a water-soluble fluorescent perylene probe. Microchim Acta 184, 2439–2444 (2017). https://doi.org/10.1007/s00604-017-2292-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2292-2