Abstract
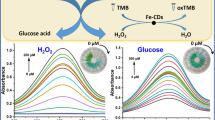
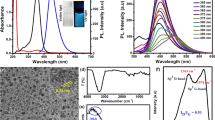
We report on a sensitive and selective fluorescent assay utilizing native carbon dots (CDs) as signal transducers. The optical probes 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) or 3,3′-diaminobenzidine (DAB) were employed as substrates of horseradish peroxidase (HRP). It was found that the corresponding oxidation products (ox-ABTS or ox-DAB) quench the fluorescence of CDs, mainly via photoinduced electron transfer in case of ox-ABTS, and via aggregation and inner filter effect in case of ox-DAB. By coupling with bienzyme (glucose oxidase and HRP)-mediated biocatalytic reactions, the method was applied to the determination of hydrogen peroxide and glucose. In case of ABTS as the substrate of HRP, a wide linear range (0.05 to 100 μM) and a very low detection limit (10 nM) for glucose were attained. The method was applied to the determination of glucose in human serum and the results were found to agree well with data provided by a local hospital.

In this work, a sensitive and selective fluorescent assay was developed for probing the enzymatic substrates of hydrogen peroxide and glucose which utilized native carbon dots (CDs) as signal transducer.








Similar content being viewed by others
References
Ro DK, Paradise EM, Quellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Guerrieri A, Palmisano F (2001) An acetylcholinesterase/choline oxidase-based amperometric biosensors as a liquid chromatography detector for acetylcholine and choline determination in brain tissue homogenates. Anal Chem 73:2875–2882
Goran JM, Lyon JL, Stevenson KJ (2011) Amperometric detection of L-lactate using nitrogen-doped carbon nanotubes modified with lactate oxidase. Anal Chem 83:8123–8129
Nakaminami T, Kuwabata S, Yoneyama H (1997) Electrochemical oxidation of cholesterol catalyzed by cholesterol oxidase with use of an artificial electron mediator. Anal Chem 69:2367–2372
Zhu WJ, Feng DQ, Chen M, Chen ZD, Zhu R, Fang HL, Wang W (2014) Bienzyme colorimetric detection of glucose with self-calibration based on tree-shaped paper strip. Sensors Actuat B-Chem 190:414–418
Gill R, Bahshi L, Freeman R, Willner I (2008) Optical detection of glucose and acetylcholine esterase inhibitors by H2O2-sensitive CdSe/ZnS quantum dots. Angew Chem Int Ed 47:1676–1679
Huang CP, Liu SW, Chen TM, Li YK (2008) A new approach for quantitative determination of glucose by using CdSe/ZnS quantum dots. Sensors Actuat B-Chem 130:338–342
Shen C, Sun YP, Wang J, Lu Y (2014) Facile route to highly photoluminescent carbon nanodots for ion detection, pH sensors and bioimaging. Nanoscale 6:9139–9147
Zuo P, Lu X, Sun Z, Guo Y, He H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183:519–542
Xiao DL, Pan RF, Li SQ, He J, Qi M, Kong SM, Gu Y, Lin R, He H (2015) Porous carbon quantum dots: one step green synthesis via L-cysteine and applications in metal ion detection. RSC Adv 5:2039–2046
Yeh TY, Chang HT (2013) Photoluminescent C-dots@RGO for sensitive detection of hydrogen peroxide and glucose. Talanta 115:718–723
Kang W, Ding Y, Zhou H, Liao Q, Yang X, Yang Y, Yang M (2015) Monitoring the activity and inhibition of alkaline phosphatase via quenching and restoration of the fluorescence of carbon dots. Microchim Acta 182:1161–1167
Shan XY, Chai LJ, Ma JJ, Qian ZS, Chen JR, Feng H (2014) B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 139:2322–2325
Zhang B, Liu CY, Liu Y (2010) A novel one-step approach to synthesize fluorescent carbon nanoparticles. Eur J Inorg Chem 28:4411–4414
Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang HF, Luo PG, Yang H, Kose ME, Chen BL, Veca LM, Xie SY (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757
Shi WB, Wang QL, Long YJ, Cheng ZL, Chen SH, Zheng HZ, Huang YM (2011) Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun 47:6695–6697
Xiong YM, Zhang YY, Rong PF, Yang J, Wang W, Liu DB (2015) A high-throughput colorimetric assay for glucose detection based on glucose oxidase-catalyzed enlargement of gold nanoparticles. Nanoscale 7:15584–15588
Feng X, Cheng HJ, Pan YW, Zheng H (2015) Development of glucose biosensors based on nanostructured graphene-conducting polyaniline composite. Biosens Bioelectron 70:411–417
Yang ZJ, Cao Y, Li J, Jian ZQ, Zhang YC, XY H (2015) Platinum nanoparticles functionalized nitrogen doped graphene platform for sensitive electrochemical glucose biosensing. Anal Chim Acta 871:35–42
Zhao CZ, Zhang ZX, Zhao Y, Yu J (2012) Photoelectrochemical detection of glucose by using an enzyme-modified photoelectrode. Chin J Chem 30:1851–1856
Zhang XY, Xu F, Zhao BQ, Ji X, Yao YW, DP W (2014) Synthesis of CdS quantum dots decorated graphene nanosheets and non-enzymatic photoelectrochemical detection of glucose. Electrochim Acta 133:615–622
Wang LL, Zheng J, Li YH, Yang S, Liu CH, Xiao Y (2014) AgNP-DNA@GQDs hybrid: new approach for sensitive detection of H2O2 and glucose via simultaneous AgNP etching and DNA cleavage. Anal Chem 86:12348–12354
Liu CH, Tseng WL (2011) Oxidase-functionalized Fe3O4 nanoparticles for fluorescence sensing of specific substrate. Anal Chem 703:87–93
Steiner M, Duerkop A, Wolfbeis O (2011) Optical methods for sensing glucose. Chem Soc Rev 40:4805–4839
Karnicka K, Miecznikowski K, Kowalewska B, Skunik M, Opallo M, Rogalski J, Schuhmann W, Kulesza PJ (2008) ABTS-modified multiwalled carbon nanotubes as an effective mediating system for bioelectrocatalytic reduction of oxygen. Anal Chem 80:7643–7648
Li T, Wang EK, Dong SJ (2010) Lead(II)-induced allosteric G-quadruplex DNAzyme as a colorimetric and chemiluminescence sensor for highly sensitive and selective Pb2+ detection. Anal Chem 82:1515–1520
Olley DA, Wren EJ, Vamvounis G, Fernee MJ, Wang X, Burn PL, Meredith P, Shaw PE (2011) Explosive sensing with fluorescent dendrimers: the role of collisional quenching. Chem Mater 23:789–794
Bano S, Mohd A, Khan AAP, Shddiqi KS (2010) Complexation and mechanism of fluorescence quenching of telmisartan with Y(III) and Nd(III). J Chem Eng Data 55:5759–5765
Zhang H, Zhang L, Liang RP, Huang J, Qiu JD (2013) Simultaneous determination of concanavalin A and peanut agglutinin by dual-color quantum dots. Anal Chem 85:10969–10976
Zhang LB, Zhu JB, Guo SJ, Li T, Li J, Wang EK (2013) Photoinduced electron transfer of DNA/Ag nanoclusters modulated by G-quadruplex/hemin complex for the construction of versatile biosensors. J Am Chem Soc 135:2403–2406
Sun K, Chen HB, Wang L, Yin SY, Wang HY, GX X (2014) Size-dependent property and cell labeling of semiconducting polymer dots. ACS Appl Mater Interfaces 6:10802–10812
Cai XC, Martin JE, Shea-Rohwer LE, Gong K, Kelley DF (2013) Thermal quenching mechanisms in II − VI semiconductor. Nanocrystals. J Phys Chem C 117:7902–7913
Seligman AM, Karnovsky MJ, Wasserkrug HL, Hanker JS (1968) Nondroplet ultra-structural demonstration of cytochrome oxidase activity with a polymerizingosmiophilic reagent, diaminobenzidine (DAB). J Cell Biol 38:1–14
Lim SY, Ahn J, Lee JS, Kim MG, Park CB (2012) Graphene-oxide-based immunosensing through fluorescence quenching by peroxidase-catalyzed polymerization. Small 8:1994–1999
Zhao TT, Bi SY, Wang Y, Wang TJ, Pang B, TT G (2014) In vitro studies on the behavior of salmeterol xinafoate and its interaction with calf thymus DNA by multi-spectroscopic techniques. Spectrochim Acta A 132:198–204
Acknowledgments
This work was supported by The National Natural Science Foundation of China (21575052,21275065), and the Fundamental Research Funds for the Central Universities (JUSRP51314B) and the Opening Foundation of Public Health Research Center at Jiangnan University (JUPH201507).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 627 kb)
Rights and permissions
About this article
Cite this article
Fang, X., Wu, XM., Hu, XL. et al. Native carbon nanodots as a fluorescent probe for assays based on the use of glucose oxidase or horseradish peroxidase. Microchim Acta 183, 2761–2770 (2016). https://doi.org/10.1007/s00604-016-1921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1921-5